













中国农业科学 ›› 2020, Vol. 53 ›› Issue (24): 5091-5103.doi: 10.3864/j.issn.0578-1752.2020.24.012
许明( ),林世强,倪冬昕,伊恒杰,刘江洪,杨志坚,郑金贵(
),林世强,倪冬昕,伊恒杰,刘江洪,杨志坚,郑金贵( )
)
收稿日期:2020-05-14
接受日期:2020-07-15
出版日期:2020-12-16
发布日期:2020-12-28
通讯作者:
郑金贵
作者简介:许明,Tel:0591-83789231;E-mail: 基金资助:
XU Ming( ),LIN ShiQiang,NI DongXin,YI HenJie,LIU JiangHong,YANG ZhiJian,ZHENG JinGui(
),LIN ShiQiang,NI DongXin,YI HenJie,LIU JiangHong,YANG ZhiJian,ZHENG JinGui( )
)
Received:2020-05-14
Accepted:2020-07-15
Online:2020-12-16
Published:2020-12-28
Contact:
JinGui ZHENG
摘要:
【目的】查尔酮合成酶(chalcone synthase,CHS)是植物类黄酮生物合成的第一个关键酶,从藤茶中克隆AgCHS1,分析其序列特征及其在藤茶中的组织表达特异性,通过体外酶活性检测和烟草遗传转化对其功能进行鉴定,为进一步研究藤茶类黄酮累积的调控机理提供理论基础。【方法】根据藤茶转录组测序结果设计引物,以藤茶叶片cDNA和基因组DNA为模板,PCR扩增得到AgCHS1。利用生物信息学方法分析该基因的序列特征,使用MEGA6和DNAMAN软件进行多重序列比对,并构建系统进化树。通过原核表达系统获得AgCHS1的重组蛋白,分析该重组蛋白对底物的催化活性,并通过高效液相色谱-质谱(HPLC-MS)对酶促反应产物进行鉴定。利用qRT-PCR技术对AgCHS1在藤茶不同器官的表达水平进行分析,并采用硝酸铝比色法测定相应的总黄酮含量变化。构建植物过量表达载体,通过叶盘法转化烟草,筛选阳性转基因后代,对T2代株系花瓣中的花青素和黄酮醇含量进行检测。【结果】AgCHS1的ORF长1 182 bp,编码393个氨基酸,基因组序列长1 315 bp,含2个外显子和1个内含子。生物信息学分析表明,AgCHS1为稳定的亲水蛋白。通过与其他物种的CHS蛋白多序列比对发现,AgCHS1含有查尔酮合成酶家族的特征序列和活性位点残基,包括丙二酰CoA结合位点和三联活性中心位点,与其他物种的CHS序列一致性较高。系统进化分析显示,AgCHS1与葡萄、山葡萄的CHS处于同一进化分支,亲缘关系最近。荧光定量PCR结果表明,AgCHS1在成熟叶和花中的表达量最高,在老叶中的表达量最低。藤茶不同器官的总黄酮含量与AgCHS1表达水平呈显著正相关。体外酶活性分析显示,重组的AgCHS1蛋白可以催化底物对-香豆酰辅酶A和丙二酰辅酶A生成柚皮素,说明该蛋白具有查尔酮合成酶活性。获得5株烟草转基因阳性株系,其中2株的花瓣颜色明显加深;与对照相比,转基因株系OE3、OE4花瓣中的花青素含量分别提高56.6%和25.3%,OE3的黄酮醇含量没有显著差异,OE4的黄酮醇含量提高39.1%。【结论】AgCHS1是藤茶中催化合成查尔酮的关键酶,过量表达AgCHS1可以提高转基因植物中花青素和黄酮醇的含量。
许明,林世强,倪冬昕,伊恒杰,刘江洪,杨志坚,郑金贵. 藤茶查尔酮合成酶基因AgCHS1的克隆及功能鉴定[J]. 中国农业科学, 2020, 53(24): 5091-5103.
XU Ming,LIN ShiQiang,NI DongXin,YI HenJie,LIU JiangHong,YANG ZhiJian,ZHENG JinGui. Cloning and Function Characterization of Chalcone Synthase Gene AgCHS1 in Ampelopsis grossedentata[J]. Scientia Agricultura Sinica, 2020, 53(24): 5091-5103.
表1
本试验中所用的引物序列"
| 引物名称 Primer name | 序列(5'-3') Primer sequence (5'-3') | 用途 Purpose |
|---|---|---|
| AgCHS1-F | GGATCCATGGTGTCGGTGCAGGAAATCAGA | ORF序列扩增、载体构建 |
| AgCHS1-R | GAGCTCTCAGTGAGCCAGTGGTGCAGAC | Amplification of ORF sequence and vector construction |
| AgCHS1-P1 | ACTCCAGCCAACTGTGTCCA | 转基因烟草的PCR鉴定 |
| AgCHS1-P2 | TGAGACCCACTTCACGCAA | PCR identification of transgenic tobacco |
| AgCHS1-qF | GCCTCAAACCCTCCGTCA | 实时荧光定量PCR Quantitative real-time PCR |
| AgCHS1-qR | GACCCACGAGTGAATCCAAGT | Quantitative real-time PCR |
| NtEF1α-qF | AATTTTGACCAAGATCGACAGG | 烟草内参基因 Control |
| NtEF1α-qR | CAGCAACAGTTTGACGCATG | Reference gene in tobacco |
| AgGAPDH-qF | CATCTCAGCCCCAAGCAA | 藤茶内参基因 |
| AgGAPDH-qR | GTGGCAGTAATGGAGTGAACAG | Reference gene in A.grossedentata |

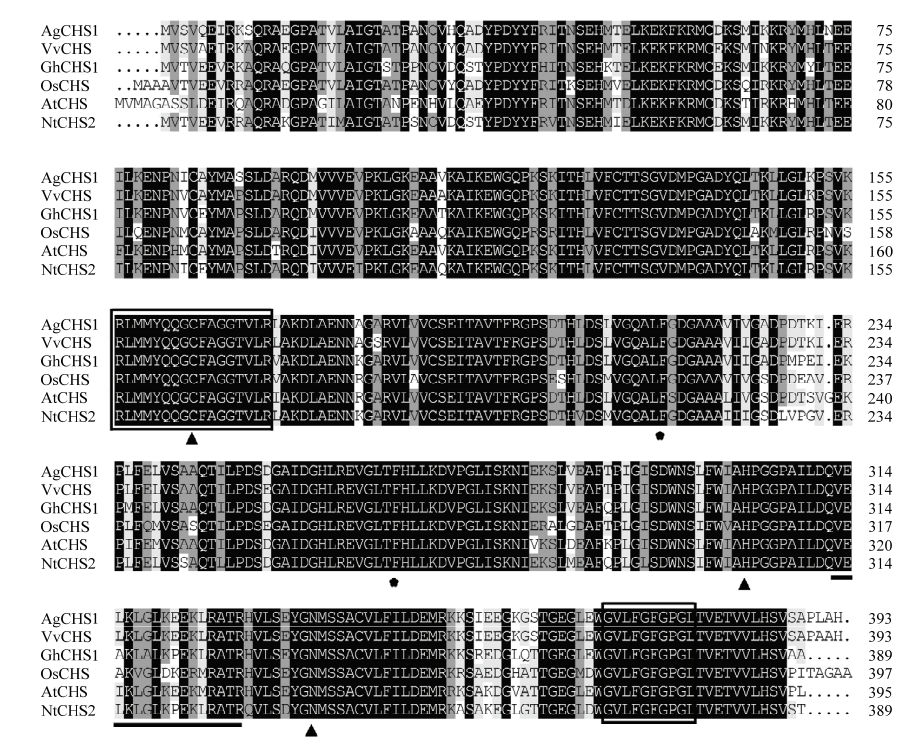
图2
AgCHS1与其他物种CHS氨基酸序列比对分析 AgCHS1:藤茶Ampelopsis grossedentata;VvCHS:葡萄 Vitis vinifera NP_001267879.1;GhCHS1:陆地棉 Gossypium hirsutum CV72638;OsCHS:水稻 Oryza sativa CAA61955.1;AtCHS:拟南芥Arabidopsis thaliana AAA32771.1;NtCHS:烟草Nicotiana tabacum ANA78328.1。▲:Cys-His-Asn三联活性中心;★:表示苯丙氨酸残基Phe215和Phe265;方框为CHS高度保守特征序列,下划线为丙二酰CoA结合位点 ▲: Cys-His-Asn triple active center; ★: phenylalanine residues Phe215 and Phe265; the highly conserved CHS signature sequence are boxed with black line; the malonyl-CoA binding site are underlined"
| [1] |
FAN L, TONG Q, DONG W W, YANG G J, HOU X L, XIONG W, SHI C Y, FANG J G, WANG W Q. Tissue distribution, excretion, and metabolic profile of dihydromyricetin, a flavonoid from Vine Tea (Ampelopsis grossedentata) after oral administration in rats. Journal of Agricultural and Food Chemistry, 2017,65(23):4597-4604.
doi: 10.1021/acs.jafc.7b01155 pmid: 28534405 |
| [2] | 何桂霞, 裴刚杜, 方麓, 欧阳文, 李斌. 藤茶化学成分的研究. 中国现代中药, 2007,9(12):11-13. |
| HE G X, PEI G D, FANG L, OUYANG W, LI B. Studies on chemical constituents of Ampelopsis grossdentata. Modern Chinese Medicine, 2007,9(12):11-13. (in Chinese) | |
| [3] | 冉京燕, 方建国, 谢雪佳, 熊微, 王文清. 藤茶的本草资源学研究概况. 中草药, 2016,47(20):3728-3735. |
| RAN J Y, FANG J G, XIE X J, XIONG W, WANG W Q. Scientific research on herbal resource of vine tea. Chinese Traditional and Herbal Drugs, 2016,47(20):3728-3735. (in Chinese) | |
| [4] | 王元霞, 洪正善, 杨柯, 曾春晖. 藤茶中二氢杨梅素的研究进展. 沈阳药科大学学报, 2020,37(6):569-576. |
| WANG Y X, HONG Z S, YANG K, ZENG C H. Research progress of dihydromyricetin in Ampelopsis grossedentata. Journal of Shenyang Pharmaceutical University, 2020,37(6):569-576. (in Chinese). | |
| [5] | 王丹丹, 王文清, 施春阳, 熊微, 侯小龙, 方建国. 藤茶中二氢杨梅素含量变异研究进展. 中药材, 2015,38(9):1996-1999. |
| WANG D D, WANG W Q, SHI C Y, XIONG W, HUO X L, FANG J G. Research progress on variation of dihydromyricetin content in Ampelopsis grossedentata. Journal of Chinese Medicinal Materials, 2015,38(9):1996-1999. (in Chinese) | |
| [6] |
TOHGE T, DE SOUZA L P, FERNIE A R. Current understanding of the pathways of flavonoid biosynthesis in model and crop plants. Journal of Experimental Botany, 2017,68(15):4013-4028.
doi: 10.1093/jxb/erx177 pmid: 28922752 |
| [7] | 包颖, 郭昌锋, 陈少华, 刘梅. 植物查尔酮合成酶超基因家族的分子进化. 植物学报, 2015,50(1):55-71. |
| BAO Y, GUO C F, CHEN S H, LIU M. Molecular evolution of chalcone synthase gene superfamily in plants. Bulletin of Botany, 2015,50(1):55-71. (in Chinese) | |
| [8] |
CHEN S, PAN X H, LI Y T, CUI L J, ZHANG Y C, ZHANG Z M, PAN G T, YANG J, CAO P J, YANG A G. Identification and characterization of chalcone synthase gene family members in Nicotiana tabacum. Journal of Plant Growth Regulation, 2017,36:374-384.
doi: 10.1007/s00344-016-9646-6 |
| [9] |
MA L Q, PANG X B, SENG Y H, PU G B, WANG H H, LEI C Y, WANG H, LI G F, LIU B Y, YE H C. A novel type III polyketide synthase encoded by a three-intron gene from Polygonum cuspidatum. Planta, 2009,229:457-469.
doi: 10.1007/s00425-008-0845-7 |
| [10] |
YAHYAA M, ALI S, DAVIDOVICH-RIKANATI R, IBDAH M, SHACHTIER A, EYAL Y, LEWINOHN E, IBDAH M. Characterization of three chalcone synthase-like genes from apple (Malus x domestica Borkh.). Phytochemistry, 2017,140:125-133.
doi: 10.1016/j.phytochem.2017.04.022 pmid: 28482241 |
| [11] |
WANG C H, ZHI S, LIU C Y, XU F X, ZHAO A C, WANG X L, TANG X, LI Z G, HUANG P H, YU M D. Isolation and characterization of a novel chalcone synthase gene family from mulberry. Plant Physiology and Biochemistry, 2017,115:107-118.
doi: 10.1016/j.plaphy.2017.03.014 pmid: 28355585 |
| [12] | 夏芳, 李厚华, 付春祥, 虞珍珍, 徐彦军, 赵德修. 水母雪莲查尔酮合酶基因的克隆、表达及酶活分析. 生物工程学报, 2011,27(9):363-370. |
| XIA F, LI H H, FU C X, YU Z Z, XU Y J, ZHAO D X. Cloning, expression and characterization of chalcone synthase from Saussurea medusa. Chinese Journal of Biotechnology, 2011,27(9):363-370. (in Chinese) | |
| [13] |
YU H N, WANG L, SUN B, GAO S, CHENG A X, LOU H X. Functional characterization of a chalcone synthase from the liverwort Plagiochasma appendiculatum. Plant Cell Reports, 2015,34(2):233-245.
doi: 10.1007/s00299-014-1702-8 pmid: 25404490 |
| [14] |
SUN W, MENG X, LIANG L, JIANG W, HUANG Y, HE J, HU H, ALMQVIST J, GAO X, WANG L. Molecular and biochemical analysis of chalcone synthase from freesia hybrid in flavonoid biosynthetic pathway. PLoS One. 2015,10(3):e0119054.
doi: 10.1371/journal.pone.0119054 pmid: 25742495 |
| [15] | 韩颖颖, 明凤. 选择与适应: 兰科植物查尔酮合酶基因家族成员的分子进化和功能趋异. 中国农业科技导报, 2012,14(4):73-80. |
| HAN Y Y, MING F. Selection and adaptation: Molecular evolution and functional divergence of orchid chalcone synthase gene family members. Journal of Agricultural Science and Technology, 2012,14(4):73-80. (in Chinese) | |
| [16] |
HU L F, HE H H, ZHU C L, PENG X S, FU J R, HE X P, CHEN X R, QUYANG L J, BIAN J M, LIU S Q. Genome‑wide identification and phylogenetic analysis of the chalcone synthase gene family in rice. Journal of Plant Research, 2017,130:95-105.
doi: 10.1007/s10265-016-0871-7 pmid: 27878652 |
| [17] |
SOMMER H, SAEDLER H. Structure of the chalcone synthase gene of Antirrhinum majus. Molecular and General Genetics, 1986,202(3):429-434.
doi: 10.1007/BF00333273 |
| [18] |
LI X H, CAO M H, MA W B, JIA C H, LI J H, ZHANG M X, LIU C C. CAO Z Z, FARUQUE M O, HU X B. Annotation of genes involved in high level of dihydromyricetin production in vine tea (Ampelopsis grossedentata) by transcriptome analysis. BMC Plant Biology, 2020,20(1):131.
doi: 10.1186/s12870-020-2324-7 pmid: 32228461 |
| [19] | WANG C K, CHEN P Y, WANG H M, TO K Y. Cosuppression of tobacco chalcone synthase using Petunia chalcone synthase construct results in white flowers. Botanical Studies, 2006,47(1):71-82. |
| [20] |
CHEN L J, GUO H M, LIN Y, CHENG H M. Chalcone synthase EaCHS1 from Eupatorium adenophorum functions in salt stress tolerance in tobacco. Plant Cell Reports, 2015,34(5):885-894.
doi: 10.1007/s00299-015-1751-7 pmid: 25632925 |
| [21] |
WANG C C, FU D Q. Virus-induced gene silencing of the eggplant chalcone synthase gene during fruit ripening modifies epidermal cells and gravitropism. Journal of Agricultural and Food Chemistry, 2018,66(11):2623-2629.
doi: 10.1021/acs.jafc.7b05617 pmid: 29494770 |
| [22] | 赵学荣, 杨燕, 雒晓鹏, 姚攀锋, 王安虎, 赵海霞, 吴琦. 苦荞查尔酮合酶基因FtCHS1启动子的克隆及分析. 植物科学学报, 2017,35(4):543-550. |
| ZHAO X R, YANG Y, LUO X P, YAO P F, WANG A H, ZHAO H X, WU Q. Cloning and analysis of chalcone synthase gene FtCHS1 promoter in Fagopyrum tataricum Gaertn. Plant Science Journal, 2017,35(4):543-550. (in Chinese) | |
| [23] | 付明, 魏麟, 余娟, 余小林. 显齿蛇葡萄查耳酮合成酶基因cDNA克隆及蛋白质序列分析. 中草药, 2013,44(1):85-89. |
| FU M, WEI L, YU J, YU X L. cDNA cloning and protein sequence analysis of chalcone synthase gene in leaves of Ampelopsis grossedentata. Chinese Traditional and Herbal Drugs, 2013,44(1):85-89. (in Chinese) | |
| [24] | 许明, 伊恒杰, 郭佳鑫, 唐进兰, 林世强, 杨志坚, 郑金贵. 藤茶黄烷酮3-羟化酶基因AgF3H的克隆及表达分析. 西北植物学报, 2020,40(2):185-192. |
| XU M, YI H J, GUO J X, TANG J L, LIN S Q, YANG Z J, ZHENG J G. Cloning and expression analysis of a flavanone 3-hydroxylase gene from Ampelopsis grossedentata. Acta Botanica Boreali-Occidentalia Sinica, 2020,40(2):185-192. (in Chinese) | |
| [25] |
LIN S Q, BI L J, ZHANG X E. A simplified method for reconstituting active E. Coli DNA polymerase Ⅲ. Protein Cell, 2011,2(4):303-307.
doi: 10.1007/s13238-011-1032-3 |
| [26] | 许明, 伊恒杰, 赵帅, 张玉文, 杨志坚, 郑金贵. 显齿蛇葡萄实时荧光定量PCR内参基因的筛选与验证. 中草药, 2017,6(3):1192-1198. |
| XU M, YI H J, ZHAO S, ZHANG Y W, YANG Z J, ZHENG J G. Screening and validation of reference genes for quantitative RT-PCR analysis in Ampelopsis grossedentata. Chinese Traditional and Herbal Drugs, 2017,6(3):1192-1198. (in Chinese) | |
| [27] |
NING G G, XIAO X, LV H Y, LI X, ZUO Y, BAO M Z. Shortening tobacco life cycle accelerates functional gene identification in genomic research. Plant Biology, 2012,14(6):934-943.
doi: 10.1111/j.1438-8677.2012.00571.x |
| [28] | 秦亚茹, 张友胜, 张凯, 丁振东, 曾萍, 孔繁晟. 藤茶总黄酮检测方法的对比研究. 现代食品科技, 2019,35(12):302-309. |
| QIN Y R, ZHANG Y S, ZHANG K, DING Z D, ZENG P, KONG F S. Comparative study of determination methods of total flavonoids in vine tea (Ampelopsis grossedentata). Modern Food Science and Technology, 2019,35(12):302-309. (in Chinese) | |
| [29] |
WANG Y, DOU Y, WANG R, GUAN X L, HU Z H, ZHENG J. Molecular characterization and functional analysis of chalcone synthase from Syringa oblata Lindl. in the flavonoid biosynthetic pathway. Gene, 2017,635:16-23.
doi: 10.1016/j.gene.2017.09.002 pmid: 28890377 |
| [30] |
TIRUMALAI V, SWETHA C, NAIR A, PANDIT A, SHIVAPRASAD P V. miR828 and miR858 regulate VvMYB114 to promote anthocyanin and flavonol accumulation in grapes. Journal of Experimental Botany, 2019,70(18):4775-4791.
doi: 10.1093/jxb/erz264 pmid: 31145783 |
| [31] | 马兰青, 师光禄, 叶和春, 刘本叶, 王有年. 植物类型Ⅲ聚酮合酶超家族基因结构、功能及代谢产物. 生物工程学报, 2010,26(11):1482-1492. |
| MA L Q, SHI G L, YE H C, LIU B Y, WANG Y N. Plant-specific type III polyketide synthase superfamily:gene structure, function and metabolistes. Chinese Journal of Biotechnology, 2010,26(11):1482-1492. (in Chinese) | |
| [32] |
张丽群, 韦康, 王丽鸳, 成浩, 刘本英, 龚武云. 茶树CHS基因结构及编码区单核苷酸多态性分析. 中国农业科学, 2014,47(1):133-144.
doi: 10.3864/j.issn.0578-1752.2014.01.014 |
|
ZHANG L Q, WEI K, WANG L Y, CHENG H, LIU B Y, GONG W Y. The structure and single nucleotide polymorphism analysis of chalcone synthase genes in tea plant (Camellia sinenesis). Scientia Agricultura Sinica, 2014,47(1):133-144. (in Chinese)
doi: 10.3864/j.issn.0578-1752.2014.01.014 |
|
| [33] |
KODURI P H, GORDON G S, BARKER E I, COLPITTS C C, ASHTON N W, SUH D Y. Genome-wide analysis of the chalcone synthase superfamily genes of Physcomitrella patens. Plant Molecular Biology, 2010,72(3):247-263.
doi: 10.1007/s11103-009-9565-z |
| [34] |
KREUZALER F, HAHLBROCK K. Enzymatic synthesis of aromatic compounds in higher plants:formation of naringenin (5,7,4’- trihydroxyflavanone) from p-coumaroyl coenzyme A and malonyl coenzyme A. FEBS Letters, 1972,28(1):69-72.
doi: 10.1016/0014-5793(72)80679-3 pmid: 4646877 |
| [35] |
JIANG C G, SCHOMMER C K, KIM S Y, SUH D Y. Cloning and characterization of chalcone synthase from the moss, Physcomitrella patens. Phytochemistry, 2006,67(23):2531-2540.
doi: 10.1016/j.phytochem.2006.09.030 pmid: 17083952 |
| [36] |
YU H N, LIU X Y, GAO S, SUN B, ZHENG H B, JI M, CHENG A X, LOU H X. Structural and biochemical characterization of the plant type III polyketide synthases of the liverwort Marchantia paleacea. Plant Physiology and Biochemistry, 2018,125:95-105.
doi: 10.1016/j.plaphy.2018.01.030 pmid: 29428820 |
| [37] |
WAKI T, MAMEDA R, NAKANO T, YAMADA S, TERASHITA M, ITO K, TENMA N, LI Y B, FUJINO N, UNO K, YAMASHITA S, AOKI Y, DENESSIOUK K, KAWAI Y, SUGAWARA S, SAITO K, YONEKURA-SAKAKIBARA K, MORITA Y, HOSHINO A, TAKAHASHI S, NAKAYAMA T. A conserved strategy of chalcone isomerase-like protein to rectify promiscuous chalcone synthase specificity. Nature Communications, 2020,11(1):870.
doi: 10.1038/s41467-020-14558-9 pmid: 32054839 |
| [38] |
MORITA H, TAKAHASHI Y, NOGUCHI H, ABE I. Enzymatic formation of unnatural aromatic polyketides by chalcone synthase. Biochemical and Biophysical Research Communications, 2000,279(1):190-195.
doi: 10.1006/bbrc.2000.3920 pmid: 11112437 |
| [39] |
李辛雷, 殷恒福, 范正琪, 李纪元. 山茶芽变花色与花青苷的关系. 中国农业科学, 2019,52(11):1961-1969.
doi: 10.3864/j.issn.0578-1752.2019.11.010 |
|
LI X L, YIN H F, FAN Z Q, LI J Y. The relationship between anthocyanins and flower colors of bud mutation in Camellia japonica. Scientia Agricultura Sinica, 2019,52(11):1961-1969. (in Chinese)
doi: 10.3864/j.issn.0578-1752.2019.11.010 |
|
| [40] |
OHNO S, HOSOKAWA M, KOJIMA M, KITAMURA Y, HOSHINO A, TATSUZAWA F, DOI M, YAZAWA S. Simultaneous post- transcriptional gene silencing of two different chalcone synthase genes resulting in pure white flowers in the octoploid dahlia. Planta, 2011, 234: 945-958.
doi: 10.1007/s00425-011-1473-1 pmid: 21735195 |
| [41] |
OHNO S, HORI W, HOSOKAWA M, TATSUZAWA F, DOI M. Post-transcriptional silencing of chalcone synthase is involved in phenotypic lability in petals and leaves of bicolor dahlia (Dahlia variabilis) ‘Yuino’. Planta, 2018, 247: 413-428.
doi: 10.1007/s00425-018-2862-5 pmid: 29455261 |
| [42] |
KAMIISHI Y, OTANI M, TAKAGI H, HAN D, MORI S, TATSUZAWA F, OKUHARA H, KOBAYASHI H, NAKANO M. Flower color alteration in the liliaceous ornamental Tricyrtis, sp. by RNA interference-mediated suppression of the chalcone synthase gene. Molecular Breeding, 2012,30(2):671-680.
doi: 10.1007/s11032-011-9653-z |
| [43] |
YUAN Y W, REBOCHO A B, SAGAWA J M, STANLEY L E, BRADSHAW, H D. Competition between anthocyanin and flavonol biosynthesis produces spatial pattern variation of floral pigments between Mimulus species. Proceedings of the National Academy of Sciences of the United States of America, 2016,113:2448-2453.
doi: 10.1073/pnas.1515294113 |
| [1] | 张家桦,杨恒山,张玉芹,李从锋,张瑞富,邰继承,周阳晨. 不同滴灌模式对东北春播玉米籽粒淀粉积累及淀粉相关酶活性的影响[J]. 中国农业科学, 2022, 55(7): 1332-1345. |
| [2] | 郭泽西,孙大运,曲俊杰,潘凤英,刘露露,尹玲. 查尔酮合成酶基因在葡萄抗灰霉病和霜霉病中的作用[J]. 中国农业科学, 2022, 55(6): 1139-1148. |
| [3] | 陈婷婷, 符卫蒙, 余景, 奉保华, 李光彦, 符冠富, 陶龙兴. 彩色稻叶片光合特征及其与抗氧化酶活性、花青素含量的关系[J]. 中国农业科学, 2022, 55(3): 467-478. |
| [4] | 王博,覃富强,邓凤莹,罗惠格,陈祥飞,成果,白扬,黄小云,韩佳宇,曹雄军,白先进. ‘阳光玫瑰’葡萄一年两收果实类黄酮组分及含量差异分析[J]. 中国农业科学, 2022, 55(22): 4473-4486. |
| [5] | 相玉婷, 王晓龙, 胡新中, 任长忠, 郭来春, 李璐. 燕麦品种间脂肪酶活性差异及低脂肪酶优质品种的预测[J]. 中国农业科学, 2022, 55(21): 4104-4117. |
| [6] | 朱长伟,孟威威,石柯,牛润芝,姜桂英,申凤敏,刘芳,刘世亮. 不同轮耕模式下小麦各生育时期土壤养分及酶活性变化特征[J]. 中国农业科学, 2022, 55(21): 4237-4251. |
| [7] | 孙保娟,汪瑞,孙光闻,王益奎,李涛,宫超,衡周,游倩,李植良. 转录组及代谢组联合解析茄子果色上位遗传效应[J]. 中国农业科学, 2022, 55(20): 3997-4010. |
| [8] | 张川,刘栋,王洪章,任昊,赵斌,张吉旺,任佰朝,刘存辉,刘鹏. 不同时期高温胁迫对夏玉米物质生产性能及籽粒产量的影响[J]. 中国农业科学, 2022, 55(19): 3710-3722. |
| [9] | 夏芊蔚,陈浩,姚宇阗,笪达,陈健,石志琦. “优标”水稻体系对稻田土壤环境的影响[J]. 中国农业科学, 2022, 55(17): 3343-3354. |
| [10] | 张英强,张水勤,李燕婷,赵秉强,袁亮. 不同含羧基有机酸改性尿素在石灰性潮土中的转化特征[J]. 中国农业科学, 2022, 55(17): 3355-3364. |
| [11] | 袁景丽,郑红丽,梁先利,梅俊,余东亮,孙玉强,柯丽萍. 花青素代谢对陆地棉叶片和纤维色泽呈现的影响[J]. 中国农业科学, 2021, 54(9): 1846-1855. |
| [12] | 郑伟,师筝,龙美,廖允成. 黄绿叶突变体冀麦5265yg的光合生理特性分析[J]. 中国农业科学, 2021, 54(21): 4539-4551. |
| [13] | 邵美琪,赵卫松,苏振贺,董丽红,郭庆港,马平. 盐胁迫下枯草芽孢杆菌NCD-2对番茄促生作用及对土壤微生物群落结构的影响[J]. 中国农业科学, 2021, 54(21): 4573-4584. |
| [14] | 郜永博,王世显,魏珉,李静,高中强,孟伦,杨凤娟. 氮磷钾用量对基质培茄子产量、根系形态和根际微生物数量与酶活性的影响[J]. 中国农业科学, 2021, 54(21): 4623-4634. |
| [15] | 任海英,周慧敏,戚行江,郑锡良,俞浙萍,张淑文,王震铄. 多效唑对杨梅土壤微生物及内生群落结构的影响[J]. 中国农业科学, 2021, 54(17): 3752-3765. |
|
||