













中国农业科学 ›› 2021, Vol. 54 ›› Issue (10): 2039-2052.doi: 10.3864/j.issn.0578-1752.2021.10.001
任志杰( ),李倩,孙钰佳,孔冬冬,刘良玉,侯聪聪(
),李倩,孙钰佳,孔冬冬,刘良玉,侯聪聪( ),李乐攻(
),李乐攻( )
)
收稿日期:2021-03-21
接受日期:2021-04-14
出版日期:2021-05-16
发布日期:2021-05-24
通讯作者:
侯聪聪,李乐攻
作者简介:任志杰,E-mail: 基金资助:
REN ZhiJie( ),LI Qian,SUN YuJia,KONG DongDong,LIU LiangYu,HOU CongCong(
),LI Qian,SUN YuJia,KONG DongDong,LIU LiangYu,HOU CongCong( ),LI LeGong(
),LI LeGong( )
)
Received:2021-03-21
Accepted:2021-04-14
Online:2021-05-16
Published:2021-05-24
Contact:
CongCong HOU,LeGong LI
摘要:
【目的】水稻花期偶遇干热风/干旱,导致脆弱的生殖细胞快速失水,极大地降低产量,这一过程中钙离子作为通用的第二信使传导了干旱或其他逆境信号,但背后的分子机制尚不清楚。分析钙离子透过性胁迫反应阳离子通道家族(calcium-permeable stress-responsive cation channels,CSCs)基因的生理和分子功能,为研究作物干热风的感应机制提供新的理论基础和思路。【方法】采用电生理学和遗传学方法,利用双电极电压钳技术在水稻中鉴定得到一个具有典型特征的受体类-钙通道蛋白,名为OsCSC11,对其蛋白序列进行生物信息学和进化关系分析。运用qRT-PCR和GUS报告基因活性分析确认OsCSC11的表达模式,在拟南芥原生质体细胞和洋葱表皮细胞中瞬时表达OsCSC11-GFP融合蛋白,验证OsCSC11的亚细胞定位;同时利用CRISPR/Cas9基因编辑技术获得OsCSC11的突变体,并通过细胞学等手段分析突变体表型和相关生理功能。【结果】蛋白序列比对发现,OsCSC11具有CSCs家族成员典型的保守结构域DUF221,但与其他成员序列差异大,存在不同于其他成员的特异结构域(motif)属于独立的亚家族。OsCSC11主要在水稻的花药和叶片中表达,进一步分析发现全长OsCSC11处于静息状态,可被高渗透溶液激活;但是删除N端156氨基酸(TM1-3)之后的OsCSC11ΔTM1-3具有组成型的通道活性,特异选择钙、镁二价阳离子;推测TM1-3是这类通道的受体结构域,感应干热风胁迫,而OsCSC11ΔTM1-3区域负责钙信号产生。OsCSC11和OsCSC11ΔTM1-3均定位在细胞质膜上,与其干热风的受体功能相适应。与野生型相比,功能缺失突变体oscsc11-1和oscsc11-2的雄蕊较小、花药表面蹙皱,整体多呈弯曲状态,花粉含水量较低,败育率高达60%—70%。【结论】OsCSC11是水稻感应短期干热风/干旱刺激、介导钙离子内流,调控花药水分状态和花粉发育的受体类钙通道,可能参与了水稻雄蕊应对干热风的原初感应过程。
任志杰,李倩,孙钰佳,孔冬冬,刘良玉,侯聪聪,李乐攻. 水稻CSC11介导干热风/干旱诱导的钙信号调控雄蕊发育[J]. 中国农业科学, 2021, 54(10): 2039-2052.
REN ZhiJie,LI Qian,SUN YuJia,KONG DongDong,LIU LiangYu,HOU CongCong,LI LeGong. OsCSC11 Mediates Dry-Hot Wind/Drought-Induced Ca2+ Signal to Regulate Stamen Development in Rice[J]. Scientia Agricultura Sinica, 2021, 54(10): 2039-2052.
表1
本研究所用的引物"
| 引物名称 Primer name | 引物序列 Primer sequence (5′-3′) | 用途 Purpose |
|---|---|---|
| CSC11-BamHⅠ-F | CGGGATCCATGGGGCCGACCGCGCCGCCGCCGGACGCCG | 蛙卵表达载体构建 Vector construction for oocyte expression |
| CSC11-EcoRⅠ-R | GGAATTCTCAGGATTGATACAGGCTCCAATCC | |
| CSC11ΔTM1-3-BamHⅠ-F | CGGGATCCATGGAGGACGCCCTTCGCA | |
| ProCSC11- Hind Ⅲ-F | CCCAAGCTTTATAGAATGGGTCATCATAGCA | p1300-proCSC11-GUS表达载体构建 Construction of of p1300-proCSC11-GUS vector |
| ProCSC11- BamHⅠ-R | CGGGATCCCGCCGGGGGACGGGGACGTGAC | |
| CSC11- EcoRⅠ-F | GGAATTCATGGGGCCGACCGCGCCGCCGCCGGACGCCG | GFP融合表达载体 GFP-CSC11 expression vector |
| CSC11-BamHⅠ-R | CGGGATCCGGATTGATACAGGCTCCAATCC | |
| CSC11ΔTM1-3-EcoRⅠ-F | GGAATTCATGGAGGACGCCCTTCGCA | |
| Target-F | GCGGCGGGGAGCCGGAGGCG | 基因敲除载体 Gene editing |
| Target-R | CGCCTCCGGCTCCCCGCCGC | |
| CSC11-CRI-F | ACCTCGCGTGATCTAGCCCCACC | 靶点检测及测序引物 Target detection and sequencing primer |
| CSC11-CRI-R | GCTTCTCTCAAGCTGGAGCTCC | |
| 11-qRT-F | GGGCATTCCCAAGACGCT | qRT-PCR检测引物 Primers used for qRT-PCR |
| 11-qRT-R | CCAAGAAATCCTGTTCCGCA | |
| OsACTIN1-F | TCCATCTTGGCATCTCTCAG | |
| OsACTIN1-R | GTACCCGCATCAGGCATCTG |
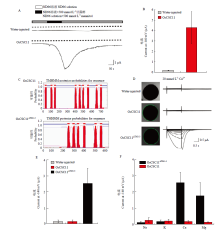
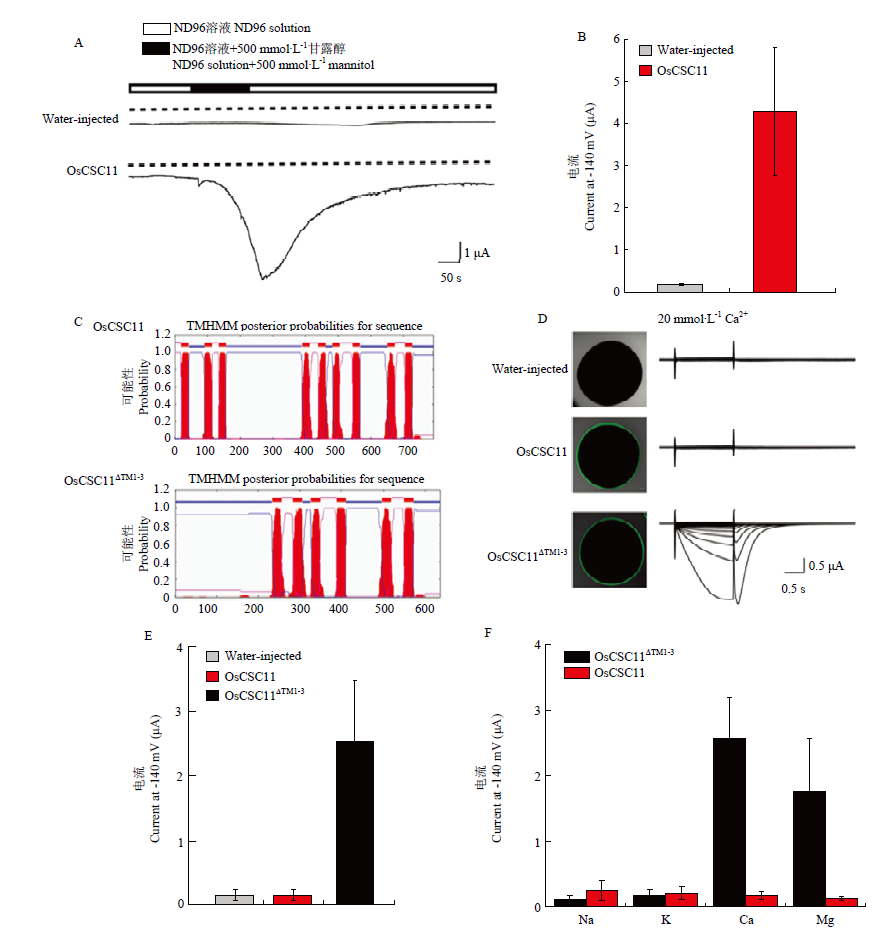
图5
OsCSC11的电生理学分析 A:全细胞记录方式检测表达OsCSC11的蛙卵和对照组注射等体积水的蛙卵在高渗胁迫时产生的电流,电压钳制在-100 mV,横坐标表示记录时间,纵坐标表示电流大小,实线表示电流示踪,虚线表示0 μA电流;B:统计分析A图中记录到的被高渗溶液激活的电流最大值,n>3;C:OsCSC11和OsCSC11ΔTM1-3蛋白的跨膜结构预测;D:OsCSC11和OsCSC11ΔTM1-3蛙卵细胞中的定位以及电生理活性检测,注射水的蛙卵作为对照;E:统计分析图B中钳制电压为-140 mV时的电流最大值,n>5;F:OsCSC11和OsCSC11ΔTM1-3的离子选择性分析,n>5;B、E和F图中的数值由平均值±标准差表示"
| [1] |
SANDERS D, BROWNLEE C, HARPER J F. Communicating with calcium. The Plant Cell, 1999,11:691-706.
doi: 10.1105/tpc.11.4.691 |
| [2] |
GILROY S, TREWAVAS A. Signal processing and transduction in plant cells: The end of the beginning? Nature Reviews Molecular Cell Biology, 2001,2(4):307-314.
doi: 10.1038/35067109 |
| [3] | LEE H J, SEO P J. Ca2+ talyzing initial responses to environmental stresses. Trends in Plant Science, 2021,8:S1360-1385. |
| [4] |
KUDLA J, BECKER D, GRILL E, HEDRICH R, HIPPLER M, KUMMER U, PARNISKE M, ROMEIS T, SCHUMACHER K. Advances and current challenges in calcium signaling. New Phytologist, 2018,218(2):414-431.
doi: 10.1111/nph.14966 |
| [5] |
SWARBRECK S M, COLAÇO R, DAVIES J M. Plant calcium- permeable channels. Plant Physiology, 2013,163(2):514-522.
doi: 10.1104/pp.113.220855 |
| [6] |
HACHEZ C, BESSERER A, CHEVALIER A S, CHAUMONT F. Insights into plant plasma membrane aquaporin trafficking. Trends in Plant Science, 2013,18(6):344-352.
doi: 10.1016/j.tplants.2012.12.003 |
| [7] |
HOU C C, TIAN W, KLEIST T, HE K, GARCIA V, BAI F, HAO Y L, LUAN S, LI L G. DUF221 proteins are a family of osmosensitive calcium-permeable cation channels conserved across eukaryotes. Cell Research, 2014,24(5):632-635.
doi: 10.1038/cr.2014.14 |
| [8] |
ZHANG M, WANG D, KANG Y, WU J X, YAO F, PAN C, YAN Z, SONG C, CHEN L. Structure of the mechanosensitive OSCA channels. Nature Structural & Molecular Biology, 2018,25(9):850-858.
doi: 10.1038/s41594-018-0117-6 |
| [9] |
MURTHY S E, DUBIN A E, WHITWAM T, JOJOA-CRUZ S, CAHALAN S M, MOUSAVI S A R, WARD A B, PATAPOUTIAN A. OSCA/TMEM63 are an evolutionarily conserved family of mechanically activated ion channels. Elife, 2018,7:e41844.
doi: 10.7554/eLife.41844 |
| [10] |
JOJOA-CRUZ S, SAOTOME K, MURTHY S E, TSUI C C A, SANSOM M S, PATAPOUTIAN A, WARD A B. Cryo-EM structure of the mechanically activated ion channel OSCA1.2. Elife, 2018,7:e41845.
doi: 10.7554/eLife.41845 |
| [11] |
LI Q, MONTELL C. Mechanism for food texture preference based on grittiness. Current Biology, 2021,31:1-12.
doi: 10.1016/j.cub.2020.09.070 |
| [12] |
DU H, YE C, WU D, ZANG Y Y, ZHANG L, CHEN C, HE X Y, YANG J J, HU P, XU Z, WAN G, SHI Y S. The cation channel TMEM63B is an osmosensor required for hearing. Cell Report, 2020,31(5):107596.
doi: 10.1016/j.celrep.2020.107596 |
| [13] | LANGRIDGE P, REYNOLDS M. Breeding for drought and heat tolerance in wheat. Theoretical and Applied Genetics, 2021, doi: 10.1007/s00122-021-03795-1. |
| [14] | LAWAS L M F, LI X, ERBAN A, KOPKA J, JAGADISH S V K, ZUTHER E, HINCHA D K. Metabolic responses of rice cultivars with different tolerance to combined drought and heat stress under field conditions. Gigascience, 2019, 8(5): giz050. |
| [15] |
VAN E S S W . Too hot to handle, the adverse effect of heat stress on crop yield. Physiologia Plantarum, 2020,169(4):499-500.
doi: 10.1111/ppl.v169.4 |
| [16] | ZHAO C, LIU B, PIAO S, WANG X, LOBELL D B, HUANG Y, HUANG M, YAO Y, BASSU S, CIAIS P, DURAND J L, ELLIOTT J, EWERT F, JANSSENS I A, LI T, LIN E, LIU Q, MARTRE P, MÜLLER C, PENG S, PEÑUELAS J, RUANE A C, WALLACH D, WANG T, WU D, LIU Z, ZHU Y, ZHU Z, ASSENG S. Temperature increase reduces global yields of major crops in four independent estimates. Proceedings of the National Academy of Sciences of the United States of America, 2017,114(35):9326-9331. |
| [17] |
ZHANG C X, FENG B H, CHEN T T, FU W M, LI H B, LI G Y, JIN Q Y, TAO L X, FU G F. Heat stress-reduced kernel weight in rice at anthesis is associated with impaired source-sink relationship and sugars allocation. Environmental and Experimental Botany, 2018,155:718-733.
doi: 10.1016/j.envexpbot.2018.08.021 |
| [18] |
ARSHAD M S, FAROOQ M, ASCH F, KRISHNA J S V, PRASAD P V V, SIDDIQUE K H M. Thermal stress impacts reproductive development and grain yield in rice. Plant Physiology and Biochemistry, 2017,115:57-72.
doi: 10.1016/j.plaphy.2017.03.011 |
| [19] |
FU G F, SONG J, XIONG J, LIAO X Y, ZHANG X F, WANG X, LE M K, TAO L X. Thermal resistance of common rice maintainer and restorer lines to high temperature during flowering and early grain filling stages. Rice Science, 2012,19:309-314.
doi: 10.1016/S1672-6308(12)60055-9 |
| [20] |
SATAKE T, YOSHIDA S. High temperature-induced sterility in Indica Rices at flowering. Japanese Journal of Crop Science, 1978,47:6-17.
doi: 10.1626/jcs.47.6 |
| [21] | SAKATA T, OSHINO T, MIURA S, TOMABECHI M, TSUNAGA Y. Auxins reverse plant male sterility caused by high temperatures. Proceedings of the National Academy of Sciences of the United States of America, 2010,107:8569-8574. |
| [22] | 曹珍珍. 高温对水稻花器伤害和籽粒品质影响的相关碳氮代谢机理[D]. 杭州: 浙江大学, 2014. |
| CAO Z Z. Mechanism of carbon and nitrogen metabolism related to effects of high temperature on floral organ injury and grain quality of rice[D]. Hangzhou: Zhejiang University, 2014. (in Chinese) | |
| [23] |
MATSUI T, OMASA K, HORIE T. High temperature-induced spikelet sterility of japonica rice at flowering in relation to air temperature, humidity and wind velocity conditions. Japanese Journal of Crop Science, 1997,66(3):449-455.
doi: 10.1626/jcs.66.449 |
| [24] |
SATAKE T, YOSHIDA S. High temperature-induced sterility in indica rice at flowering. Japanese Journal of Crop Science, 2011,47(1):6-17.
doi: 10.1626/jcs.47.6 |
| [25] |
MATSUI T, OMASA K, HORIE T. The difference in sterility due to high temperatures during the flowering period among japonica-rice varieties. Plant Production Science, 2001,4(2):90-93.
doi: 10.1626/pps.4.90 |
| [26] |
ZHAI Y, WEN Z, HAN Y, ZHUO W, WANG F, XI C, LIU J, GAO P, ZHAO H, WANG Y, WANG Y, HAN S. Heterogeneous expression of plasma-membrane-localised OsOSCA1.4 complements osmotic sensing based on hyperosmolality and salt stress in Arabidopsis osca1 mutant. Cell Calcium, 2020,91:102261.
doi: 10.1016/j.ceca.2020.102261 |
| [27] |
LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method . Methods, 2001,25:402-408.
doi: 10.1006/meth.2001.1262 |
| [28] | KOMARI T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant Journal for Cell & Molecular Biology, 2010,6:271-282. |
| [29] |
JEFFERSON R A. The GUS reporter gene system. Nature, 1989,342:837-838.
doi: 10.1038/342837a0 |
| [30] |
YOO S D, CHO Y H, SHEEN J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nature Protocol, 2007,2:1565-1572.
doi: 10.1038/nprot.2007.199 |
| [31] |
EADY C, WELD R, LISTER C. Agrobacterium tumefaciens- mediated transformation and regeneration of onion (Allium cepa L.). Plant Cell Report, 2000,19:376-381.
doi: 10.1007/s002990050743 |
| [32] |
ZHANG S S, PAN Y J, TIAN W, DONG M Q, ZHU H F, LUAN S, LI L G. Arabidopsis CNGC14 mediates calcium influx required for tip growth in root hairs. Molecular Plant, 2017,10(7):1004-1006.
doi: 10.1016/j.molp.2017.02.007 |
| [33] |
PAN Y J, CHAI X Y, GAO Q F, ZHOU L M, ZHANG S S, LI L G, LUAN S. Dynamic interactions of plant CNGC subunits and calmodulins drive oscillatory Ca2+ channel activities . Developmental Cell, 2019,48(5):710-725.
doi: 10.1016/j.devcel.2018.12.025 |
| [34] |
TIAN W, HOU C C, REN Z J, WANG C, ZHAO F G, DAHLBECK D, HU S, ZHANG L Y, NIU Q, LI L G, STASKAWICZ B J, LUAN S. A calmodulin-gated calcium channel links pathogen patterns to plant immunity. Nature, 2019,572(7767):131-135.
doi: 10.1038/s41586-019-1413-y |
| [35] |
KUDLA J, BATISTIC O, HASHIMOTO K. Calcium signals: The lead currency of plant information processing. The Plant Cell, 2010,22(3):541-563.
doi: 10.1105/tpc.109.072686 |
| [36] |
BERRIDGE M J, LIPP P, BOOTMAN M D. The versatility and universality of calcium signalling. Nature Reviews Molecular Cell Biology, 2000,1:11-21.
doi: 10.1038/35036035 |
| [37] |
DAVENPORT R. Glutamate receptors in plants. Annals of Botany, 2002,90(5):549-557.
doi: 10.1093/aob/mcf228 |
| [38] |
JHA S K, SHARMA M, PANDEY G K. Role of cyclic nucleotide gated channels in stress management in plants. Current Genomics, 2016,17(4):315-329.
doi: 10.2174/1389202917666160331202125 |
| [39] | FORDE B G, ROBERTS M R. Glutamate receptor-like channels in plants: A role as amino acid sensors in plant defence? F1000Prime Reports, 2014,6:37. |
| [40] |
WANG X H, FENG C X, TIAN L L, HOU C C, TIAN W, HU B, ZHANG Q, REN Z J, NIU Q, SONG J L, KONG D D, LIU L Y, HE Y K, MA L G, CHU C C, LUAN S, LI L G. A transceptor-channel complex couples nitrate sensing to calcium signaling in Arabidopsis. Molecular Plant, 2021,14(5):774-786.
doi: 10.1016/j.molp.2021.02.005 |
| [41] |
YUAN F, YANG H, XUE Y, KONG D, YE R, LI C, ZHANG J, THEPRUNGSIRIKUL L, SHRIFT T, KRICHILSKY B, JOHNSON D M, SWIFT G B, HE Y, SIEDOW J N, PEI Z M. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature, 2014,514(7522):367-371.
doi: 10.1038/nature13593 |
| [42] | DENIS V, CYERT M S. Internal Ca2+ release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. Journal of Cell Biology, 2002,156:29-34. |
| [43] |
CATERINA M J, SCHUMACHER M A, TOMINAGA M, ROSEN T A, LEVINE J D, JULIUS D. The capsaicin receptor: A heat- activated ion channel in the pain pathway. Nature, 1997,389(6653):816-824.
doi: 10.1038/39807 |
| [44] |
PEIER A M, MOQRICH A, HERGARDEN A C, REEVE A J, ANDERSSON D A, STORY G M, EARLEY T J, DRAGONI I, MCINTYRE P, BEVAN S, PATAPOUTIAN A. A TRP channel that senses cold stimuli and menthol. Cell, 2002,108(5):705-715.
doi: 10.1016/S0092-8674(02)00652-9 |
| [45] |
MCKEMY D D, NEUHAUSSER W M, JULIUS D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature, 2002,416(6876):52-58.
doi: 10.1038/nature719 |
| [46] |
VANNESTE M, SEGAL A, VOETS T, EVERAERTS W. Transient receptor potential channels in sensory mechanisms of the lower urinary tract. Nature Reviews Urology, 2021,18(3):139-159.
doi: 10.1038/s41585-021-00428-6 |
| [47] | ZHOUX L, BATIZA A F, LOUKIN S H, PALMER C P, KUNG C, SAIMI Y. The transient receptor potential channel on the yeast vacuole is mechanosensitive. Proceedings of the National Academy of Sciences of the United States of America, 2003,100(12):7105-7110. |
| [48] |
ZHANG Y F, MARK A H, JAYARAM C, KEN L MUELLER, BOAZ C, WU D Q, CHARLES S ZUKER, NICHOLAS J P R. Coding of sweet, bitter, and umami tastes: Different receptor cells sharing similar signaling pathways. Cell, 2003,112:293-301.
doi: 10.1016/S0092-8674(03)00071-0 |
| [1] | 肖德顺, 徐春梅, 王丹英, 章秀福, 陈松, 褚光, 刘元辉. 水培条件下根际氧环境对水稻幼苗磷吸收的影响及其生理机制[J]. 中国农业科学, 2023, 56(2): 236-248. |
| [2] | 张晓丽, 陶伟, 高国庆, 陈雷, 郭辉, 张华, 唐茂艳, 梁天锋. 直播栽培对双季早稻生育期、抗倒伏能力及产量效益的影响[J]. 中国农业科学, 2023, 56(2): 249-263. |
| [3] | 桑世飞,曹梦雨,王亚男,王君怡,孙晓涵,张文玲,姬生栋. 水稻氮高效相关基因的研究进展[J]. 中国农业科学, 2022, 55(8): 1479-1491. |
| [4] | 韩晓彤,杨保军,李苏炫,廖福兵,刘淑华,唐健,姚青. 基于图像的水稻纹枯病智能测报方法[J]. 中国农业科学, 2022, 55(8): 1557-1567. |
| [5] | 赵凌, 张勇, 魏晓东, 梁文化, 赵春芳, 周丽慧, 姚姝, 王才林, 张亚东. 利用高密度Bin图谱定位水稻抽穗期剑叶叶绿素含量QTL[J]. 中国农业科学, 2022, 55(5): 825-836. |
| [6] | 蒋晶晶,周天阳,韦陈华,邬佳宁,张耗,刘立军,王志琴,顾骏飞,杨建昌. 不同栽培措施对超级稻强、弱势粒品质的影响[J]. 中国农业科学, 2022, 55(5): 874-889. |
| [7] | 张亚玲, 高清, 赵羽涵, 刘瑞, 付忠举, 李雪, 孙宇佳, 靳学慧. 黑龙江省水稻种质稻瘟病抗性评价及抗瘟基因结构分析[J]. 中国农业科学, 2022, 55(4): 625-640. |
| [8] | 陈婷婷, 符卫蒙, 余景, 奉保华, 李光彦, 符冠富, 陶龙兴. 彩色稻叶片光合特征及其与抗氧化酶活性、花青素含量的关系[J]. 中国农业科学, 2022, 55(3): 467-478. |
| [9] | 赫磊,路凯,赵春芳,姚姝,周丽慧,赵凌,陈涛,朱镇,赵庆勇,梁文化,王才林,朱丽,张亚东. 水稻穗顶端退化突变体paa21的表型分析及基因克隆[J]. 中国农业科学, 2022, 55(24): 4781-4792. |
| [10] | 杜文婷,雷肖肖,卢慧宇,王云凤,徐佳星,罗彩霞,张树兰. 氮肥减量施用对我国三大粮食作物产量的影响[J]. 中国农业科学, 2022, 55(24): 4863-4878. |
| [11] | 赵春芳,赵庆勇,吕远大,陈涛,姚姝,赵凌,周丽慧,梁文化,朱镇,王才林,张亚东. 半糯粳稻品种核心标记的筛选及DNA指纹图谱的构建[J]. 中国农业科学, 2022, 55(23): 4567-4582. |
| [12] | 刘淑军,李冬初,黄晶,刘立生,吴丁,李照全,吴远帆,张会民. 水稻油菜轮作下稻草还田和钾肥对土壤团聚体及钾素分布的影响[J]. 中国农业科学, 2022, 55(23): 4651-4663. |
| [13] | 刘进,胡佳晓,马小定,陈武,勒思,Jo Sumin,崔迪,周慧颖,张立娜,Shin Dongjin,黎毛毛,韩龙植,余丽琴. 水稻RIL群体高密度遗传图谱的构建及苗期耐热性QTL定位[J]. 中国农业科学, 2022, 55(22): 4327-4341. |
| [14] | 万华琴,辜旭,何红梅,汤逸帆,申建华,韩建刚,朱咏莉. 沼液中HCO3-对水稻生长的类CO2施肥效应[J]. 中国农业科学, 2022, 55(22): 4445-4457. |
| [15] | 逄洪波, 程露, 于茗兰, 陈强, 李玥莹, 吴隆坤, 王泽, 潘孝武, 郑晓明. 栽培稻芽期耐低温全基因组关联分析[J]. 中国农业科学, 2022, 55(21): 4091-4103. |
|
||