













中国农业科学 ›› 2020, Vol. 53 ›› Issue (22): 4571-4583.doi: 10.3864/j.issn.0578-1752.2020.22.005
吕楚阳1,邓平川2,张晓丽1,孙钰超1,梁五生1,胡东维1( )
)
收稿日期:2020-03-23
接受日期:2020-04-25
出版日期:2020-11-16
发布日期:2020-11-28
通讯作者:
胡东维
作者简介:吕楚阳,E-mail:基金资助:
LÜ ChuYang1,DENG PingChuan2,ZHANG XiaoLi1,SUN YuChao1,LIANG WuSheng1,HU DongWei1( )
)
Received:2020-03-23
Accepted:2020-04-25
Online:2020-11-16
Published:2020-11-28
Contact:
DongWei HU
摘要: 【目的】近40年来,稻曲病在世界各主要水稻栽培区均表现出发生规模不断扩大、严重程度不断增加的趋势,并逐渐发展成为水稻主要病害之一。前期对低温诱导初期的稻曲球进行切片,发现稻曲球内含有大量隐含菌核。本研究通过转录组高通量测序鉴定低温诱导稻曲病菌(Villosiclava virens)菌核形成的潜在调控基因,阐释菌核形成的分子机制,为揭示稻曲病发生规律及有效防治打下基础。【方法】利用高通量测序技术,对低温诱导处理的稻曲球进行转录组测序(对照组:TL911_1、TL911_2、TL911_3;低温处理组:FH1016_1、FH1016_2、FH1016_3)。以稻曲病菌基因组(UV-8b)作为参考基因组进行序列比对,利用FPKM法计算基因表达量,设定参数(|log2 fold change|≥1且q-value≤0.05)筛选差异表达基因。结合基因差异表达分析、基因家族分析和富集分析(Gene Ontology/KEGG Pathway),鉴定稻曲病菌菌核形成关键基因,并利用实时荧光定量PCR(qRT-PCR)技术对其表达量进行验证。【结果】转录组测序共获得59.78 G高质量数据,其中,近93.2%的数据能够比对到稻曲病菌基因组。数据分析共鉴定到8 426个基因存在不同程度表达,占总体基因的97.13%。与对照相比,低温处理可诱导793个基因显著差异表达,分别有398和395个基因表现为上调、下调表达,随机挑选6个基因进行qRT-PCR验证,试验结果与转录组分析一致。在差异表达基因中,共注释到180个(22.7%)基因家族,其中61.67%的基因家族表现为上调表达,主要包括MFS转运蛋白、糖转运蛋白、锌指转录因子等。GO富集分析发现,差异表达基因显著富集于碳水化合物代谢、氧化还原过程、氧化还原酶活性等。KEGG分析发现,差异表达基因显著富集于次生代谢产物生物合成、淀粉蔗糖代谢、糖酵解/糖异生等代谢通路,暗示营养物质代谢和能量代谢途径相关基因表达对于低温诱导菌核形成至关重要。【结论】低温可诱导稻曲病菌菌核形成。低温使机体内部处于氧化应激状态,可通过信号转导途径放大,该过程由多个基因参与并调控多个基因家族成员,最终促使跨膜运输、细胞形态、生物合成等基因的上调表达,使得在形成菌核过程中蛋白表达活跃,达到合成细胞及物质的高峰期,进而促进菌核形成。
吕楚阳,邓平川,张晓丽,孙钰超,梁五生,胡东维. 低温诱导稻曲病菌菌核形成的转录组学分析[J]. 中国农业科学, 2020, 53(22): 4571-4583.
LÜ ChuYang,DENG PingChuan,ZHANG XiaoLi,SUN YuChao,LIANG WuSheng,HU DongWei. Transcriptomic Analysis of Sclerotia Formation Induced by Low Temperature in Villosiclava virens[J]. Scientia Agricultura Sinica, 2020, 53(22): 4571-4583.
表1
实时荧光定量PCR所用引物"
| 序号No. | 蛋白名称 Protein name | 蛋白ID Protein ID | 引物序列 Primer sequence (5′-3′) | 扩增长度 Amplicon size (bp) |
|---|---|---|---|---|
| 1 | Protein rds1 | KDB11131.1 | F: TCCAACGTGCGGGAATACA | 164 |
| R: CCAAACTGGCGGAAAATC | ||||
| 2 | Biotrophy-associated secreted protein 2 | KDB17523.1 | F: GCCTCAGCAACACCGACT | 220 |
| R: GCCTTTACCTCGTCCGCC | ||||
| 3 | Glycoside hydrolase | KDB17641.1 | F: TCCTCGCCACCATCTCGT | 178 |
| R: CGCCTCATCGCCCTCAAC | ||||
| 4 | Cytosolic phospholipase A2 zeta | KDB15918.1 | F: ATGGGCGTCTTTGGGAGCG | 165 |
| R: GGGAATTGTGGCGGGATCT | ||||
| 5 | CRE-TRX-1 protein | KDB18233.1 | F: GCCAAATCCCGACAAAAG | 142 |
| R: GGCGGCGTAGTCACCATA | ||||
| 6 | Methylenetetrahydrofolate reductase 1 | KDB13169.1 | F: TCCTCGCCACCATCTCGTC | 179 |
| R: CCGCCTCATCGCCCTCAAC | ||||
| 7 | α-tubulin | KDB12764.1 | F: GCTCTCGTGCTTGCTCTTGG | 144 |
| R: ATCACTTCGTCCTTGCGTTT |
表2
转录组测序数据以及与参考基因组比对结果"
| 样品 Sample | 总数据 Total reads | 比对数据 Mapped reads | 比对率 Mapped ratio (%) | 有效序列 Clean bases (G) | GC含量 GC content (%) | Q30 (%) |
|---|---|---|---|---|---|---|
| TL911_1 | 59349736 | 55846678 | 94.10 | 8.90 | 57.15 | 93.51 |
| TL911_2 | 59738802 | 55976056 | 93.70 | 8.96 | 56.95 | 93.09 |
| TL911_3 | 72656794 | 68033430 | 93.64 | 10.90 | 56.85 | 93.01 |
| FH1016_1 | 59387684 | 54809829 | 92.29 | 8.91 | 57.09 | 92.45 |
| FH1016_2 | 63542726 | 58890927 | 92.68 | 9.53 | 57.25 | 93.53 |
| FH1016_3 | 83852442 | 77646536 | 92.60 | 12.58 | 57.22 | 93.53 |
| 总计Total | 398528184 | 371203456 | - | 59.78 | - | - |
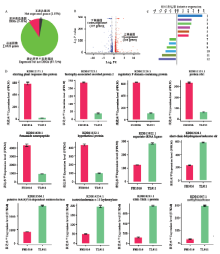
图3
低温处理下稻曲病菌表达差异显著基因的比较分析A:表达差异显著基因比例The proportion of DEGs;B:低温处理下稻曲病菌表达显著上调和下调基因分布The distribution of up- and down-regulated expression genes in V. virens under low temperature;C:前6位上、下调表达差异显著基因的相对表达量Relative expression of top-6 up- and down-regulated DEGs。1: Eliciting plant response-like protein; 2: Biotrophy-associated secreted protein 2; 3: Regulatory P domain-containing protein; 4: Protein rds1; 5: Fvmamide neuropeptide; 6: Hypothetical protein; 7: Aspartate-tRNA ligase; 8: Short-chain dehydrogenase/reductase sdr; 9: Putative NAD(P)H-dependent oxidoreductase; 10: Isotrichodermin c-15 hydroxylase; 11: CRE-TRX-1 protein; 12: Methyltransferase;D:前6位上、下调表达差异显著基因的FPKM表达量FPKM expression of top-6 up- and down-regulated DEGs"
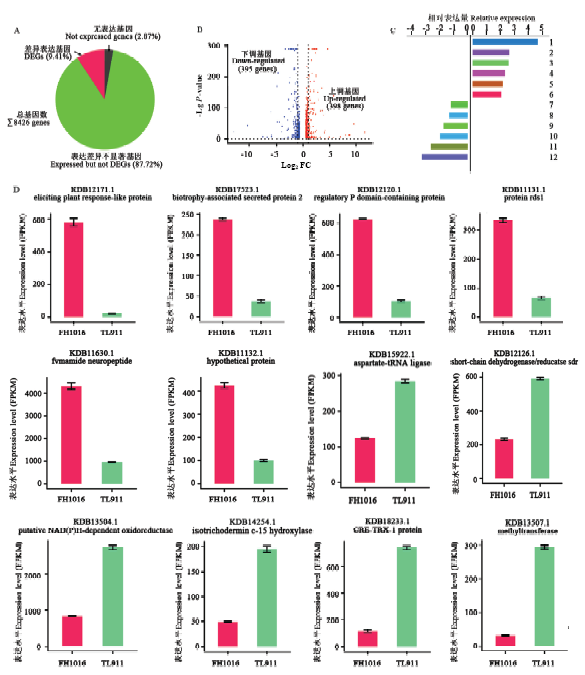
表3
差异表达基因在KEGG代谢通路中显著富集分析"
| 途径ID Pathway ID | 途径描述 Description of pathway | DEG数量 DEGs number | 上调数量 Up-regulated number | 下调数量 Down-regulated number | P值 P-value |
|---|---|---|---|---|---|
| fgr01110 | 次生代谢产物生物合成Biosynthesis of secondary metabolites | 174 | 119 | 55 | 0.000456851 |
| fgr00500 | 淀粉和蔗糖代谢Starch and sucrose metabolism | 25 | 20 | 5 | 0.001216228 |
| fgr00010 | 糖酵解/糖异生Glycolysis/Gluconeogenesis | 23 | 17 | 6 | 0.002826092 |
| fgr01200 | 碳代谢Carbon metabolism | 64 | 42 | 22 | 0.003358729 |
| fgr00680 | 甲烷代谢Methane metabolism | 15 | 10 | 5 | 0.004476103 |
| fgr01130 | 抗生素的生物合成Biosynthesis of antibiotics | 128 | 86 | 42 | 0.004815518 |
| fgr00250 | 丙氨酸、天冬氨酸和谷氨酸代谢Alanine, aspartate and glutamate metabolism | 19 | 13 | 6 | 0.006041910 |
| fgr00520 | 氨基糖和核苷酸糖代谢Amino sugar and nucleotide sugar metabolism | 27 | 15 | 12 | 0.006396843 |
| [1] |
FAN J, YANG J, WANG Y Q, LI G B, LI Y, HUANG F, WANG W M.Current understanding on Villosiclava virens, a unique flower- infecting fungus causing rice false smut disease. Molecular Plant Pathology, 2016, 17(9): 1321-1330.
doi: 10.1111/mpp.12362 pmid: 26720072 |
| [2] | TANAKA E, ASHIZAWA T, SONODA R, TANANKA C. Villosiclava virens gen. nov., comb. nov., teleomorph of Ustilaginoidea virens, the causal agent of rice false smut. Mycotaxon, 2008, 106: 491-501. |
| [3] | YAMAMOTO S, YPSHINO J.Storage period of sclerotia of Ustilaginoidea virens. Annual Report of the Kanto-Tosan Plant Protection Society, 1955, 2: 10. |
| [4] | YAEGASHI H, FUJITA Y, SONODA R.Severe outbreak of false smut of rice in 1988.Plant Protection Tokyo, 1989, 43(6): 311-314. |
| [5] | FUJITA Y, SONODA R, YAEGASHI H.The fruiting body formation in the sclerotia of Ustilaginoidea viren and the effect by light. Annual Report of the Society of Plant Protection of North Japan, 1990, 41: 205. |
| [6] | 缪巧明. 稻曲病菌核的研究. 云南农业大学学报, 1994, 9(2): 101-104. |
| MIAO Q M.Studies on the sclerotium ofUstilaginoidea viren (Cooke) Tak. Journal of Yunnan Agricultural University, 1994, 9(2): 101-104. (in Chinese) | |
| [7] | SINGH R A, DUBE K S.Occurrence of true sclerotia in Claviceps oryzae-sativae—The causal organism of false smut of rice. Current Science, 1976, 45(21): 772-773. |
| [8] | 董珂, 傅淑云. 水稻稻曲病菌菌核萌发试验初报. 沈阳农业大学学报, 1989, 20(3): 359-362. |
| DONG K, FU S Y.Sprouting test on sclerotia of rice false smut.Journal of Shenyang Agricultural University, 1989, 20(3): 359-362. (in Chinese) | |
| [9] | 黎毓干, 康必鉴, 张宝棣, 蓝毓涛, 曾红蕾, 马慧坤, 谢考祥, 李廷芳. 稻曲病研究初报. 广东农业科学, 1986(4): 45-47. |
| LI Y G, KANG B J, ZHANG B D, LAN Y T, ZENG H L, MA H K, XIE K X, LI T F.Primary studies on rice false smut.Guangdong Agricultural Sciences, 1986(4): 45-47. (in Chinese) | |
| [10] | 金敏忠, 黎煜. 水稻稻曲病菌菌核田间发生情况调查. 浙江农业科学, 1987(5): 238-239. |
| JIN M Z, LI Y.Survey on the sclerotia ofUstilaginoidea virens in the paddy field. Journal of Zhejiang Agricultural Sciences, 1987(5): 238-239. (in Chinese) | |
| [11] |
FAN L L, YONG M L, LI D Y, LIU Y J, LAI C H, CHEN H M, CHENG F M, HU D W.Effect of temperature on the development of sclerotia in Villosiclava virens. Journal of Integrative Agriculture, 2016, 15(11): 2550-2555.
doi: 10.1016/S2095-3119(16)61400-4 |
| [12] | 李丹阳, 邓启得, 雍明丽, 王华, 赖朝辉, 陈宏明, 何文苗, 胡东维. 稻曲病菌菌核降解微生物的筛选与作用机制分析. 中国生物防治学报, 2016, 32(2): 258-264. |
| LI D Y, DENG Q D, YONG M L, WANG H, LAI C H, CHEN H M, HE W M, HU D W.Screening of biocontrol fungi against sclerotia ofVillosiclava virens and their mechanisms. Chinese Journal of Biological Control, 2016, 32(2): 258-264. (in Chinese) | |
| [13] |
YU J J, YU M N, NIE Y F, SUN W X, YIN X L, ZHAO J, WANG Y H, DING H, QI Z Q, DU Y, HUANG L, LIU Y F.Comparative transcriptome analysis of fruiting body and sporulating mycelia of Villosiclava virens reveals genes with putative functions in sexual reproduction. Current Genetics, 2016, 62(3): 575-584.
doi: 10.1007/s00294-015-0563-1 pmid: 26905382 |
| [14] | 韩彦卿, 韩渊怀, 张春来, 孙文献. 水稻幼穗与Ustilaginoidea virens互作早期的转录组分析. 植物病理学报, 2019, 49(3): 296-305. |
| HAN Y Q, HAN Y H, ZHANG C L, SUN W X.Transcriptomic analysis of early interaction between rice young spikelets andUstilaginoidea virens. Acta Phytopathologica Sinica, 2019, 49(3): 296-305. (in Chinese) | |
| [15] |
PERTEA M, KIM D, PERTEA G M, LEEK J T, SALZBERG S L.Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown.Nature Protocols, 2016, 11(9): 1650-1667.
doi: 10.1038/nprot.2016.095 pmid: 27560171 |
| [16] |
TRAPNELL C, WILLAMS B A, PERTEA G, MORTAZAVI A, KWAN G, BAREN M J, SALZBERG S L, WOLD B J, PACHTER L.Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation.Nature Biotechnology, 2010, 28(5): 511-515.
doi: 10.1038/nbt.1621 pmid: 20436464 |
| [17] |
LIVAK K J, SCHMITTGEN T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method.Methods, 2001, 25(4): 402-408.
doi: 10.1006/meth.2001.1262 pmid: 11846609 |
| [18] |
PAPAPOSTOLOU I, GEORGIOU C D.Superoxide radical is involved in the sclerotial differentiation of filamentous phytopathogenic fungi: Identification of a fungal xanthine oxidase. Fungal Biology, 2010, 114(5/6): 387-395.
doi: 10.1016/j.funbio.2010.01.010 |
| [19] |
PAPAPOSTOLOU I, SIDERI M, GEORGIOU C D.Cell proliferating and differentiating role of H2O2 in Sclerotium rolfsii and Sclerotinia sclerotiorum. Microbiological Research, 2014, 169(7/8): 527-532.
doi: 10.1016/j.micres.2013.12.002 |
| [20] |
HARRIS S D.Cdc42/Rho GTPases in fungi: Variations on a common theme.Molecular Microbiology, 2011, 79(5): 1123-1127.
doi: 10.1111/j.1365-2958.2010.07525.x |
| [21] |
KOCH A, KUMAR N, WEBER L, KELLER H, IMANI J, KOGEL K H.Host-induced gene silencing of cytochrome P450 lanosterol C14 alpha-demethylase-encoding genes confers strong resistance to Fusarium species. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(48): 19324-19329.
doi: 10.1073/pnas.1306373110 |
| [22] |
EOM T J, MOON H, YU J H, PARK H S.Characterization of the velvet regulators inAspergillus flavus. Journal of Microbiology, 2018, 56(12): 893-901.
doi: 10.1007/s12275-018-8417-4 |
| [23] |
BAYRAM O, KRAPPMANN S, NI M, BOK J W, HELMSTAEDT K, VALERIUS O, BRAUS-STROMEYER S, KWON N J, KELLER N P, YU J H, BRAUS G H.VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism.Science, 2008, 320(5882): 1504-1506.
doi: 10.1126/science.1155888 pmid: 18556559 |
| [24] |
GEORGIOU C D.Lipid peroxidation in Sclerotium rolfsii: A new look into the mechanism of sclerotial biogenesis in fungi. Mycological Research, 1997, 101(4): 460-464.
doi: 10.1017/S0953756296002882 |
| [25] |
GEORGIOU C D, PATSOUKIS N, PAPAPOSTOLOU I, ZERVOUDAKIS G.Sclerotial metamorphosis in filamentous fungi is induced by oxidative stress.Integrative and Comparative Biology, 2006, 46(6): 691-712.
doi: 10.1093/icb/icj034 pmid: 21672779 |
| [26] |
PAPAPOSTOLOU I, GEORGIOU C D.Hydrogen peroxide is involved in the sclerotial differentiation of filamentous phytopathogenic fungi.Journal of Applied Microbiology, 2010, 109(6): 1929-1936.
doi: 10.1111/j.1365-2672.2010.04822.x pmid: 20681971 |
| [27] |
KIM H J, CHEN C B, KABBAGE M, DICKMAN M B.Identification and characterization of Sclerotinia sclerotiorum NADPH oxidases. Applied and Environmental Microbiology, 2011, 77(21): 7721-7729.
doi: 10.1128/AEM.05472-11 |
| [28] |
SEGMULLER N, KOKKELINK L, GIESBERT S, ODINIUS D, VAN KAN J, TUDZYNSKI P.NADPH oxidases are involved in differentiation and pathogenicity in Botrytis cinerea. Molecular Plant-Microbe Interactions, 2008, 21(6): 808-819.
doi: 10.1094/MPMI-21-6-0808 pmid: 18624644 |
| [29] |
ANGELOVA M B, PASHOVA S B, SPASOVA B K, VASSILEV S V, SLOKOSKA L S.Oxidative stress response of filamentous fungi induced by hydrogen peroxide and paraquat.Mycological Research, 2005, 109(2): 150-158.
doi: 10.1017/S0953756204001352 |
| [30] |
GRINTZALIS K, VERNARDIS S I, KLAPA M I, GEORGIOU C D.Role of oxidative stress in sclerotial differentiation and aflatoxin B1 biosynthesis in Aspergillus flavus. Applied and Environmental Microbiology, 2014, 80(18): 5561-5571.
doi: 10.1128/AEM.01282-14 |
| [31] |
YAP H Y Y, CHOOI Y H, FUNG S Y, NG S T, TAN C S, TAN N H. Transcriptome analysis revealed highly expressed genes encoding secondary metabolite pathways and small cysteine-rich proteins in the sclerotium of Lignosus rhinocerotis. PLoS ONE, 2015, 10(11): e0143549.
doi: 10.1371/journal.pone.0143549 pmid: 26606395 |
| [32] |
KIM H R, CHAE K S, HAN K H, HAN D M.The nsdC gene encoding a putative C2H2-type transcription factor is a key activator of sexual development in Aspergillus nidulans. Genetics, 2009, 182(3): 771-783.
doi: 10.1534/genetics.109.101667 pmid: 19416940 |
| [33] |
DURAN R M, CARY J W, CALVO A M.Production of cyclopiazonic acid, aflatrem, and aflatoxin by Aspergillus flavusis regulated by veA, a gene necessary for sclerotial formation. Applied Microbiology and Biotechnology, 2007, 73(5): 1158-1168.
doi: 10.1007/s00253-006-0581-5 |
| [34] |
AMAIKE S, KELLER N P.Distinct roles for VeA and LaeA in development and pathogenesis of Aspergillus flavus. Eukaryotic Cell, 2009, 8(7): 1051-1060.
doi: 10.1128/EC.00088-09 pmid: 19411623 |
| [35] |
KIM H S, HAN K Y, KIM K J, HAN D M, JAHNY K Y, CHAE K S.TheveA gene activates sexual development in Aspergillus nidulans. Fungal Genetics and Biology, 2002, 37(1): 72-80.
doi: 10.1016/S1087-1845(02)00029-4 |
| [1] | 王俊娟,陆许可,王延琴,王帅,阴祖军,付小琼,王德龙,陈修贵,郭丽雪,陈超,赵兰杰,韩迎春,孙亮庆,韩明格,张悦新,范亚朋,叶武威. 陆地棉遗传标准系TM-1的特性及其耐冷性[J]. 中国农业科学, 2022, 55(8): 1503-1517. |
| [2] | 董桑婕,姜小春,王羚羽,林锐,齐振宇,喻景权,周艳虹. 远红光补光对辣椒幼苗生长和非生物胁迫抗性的影响[J]. 中国农业科学, 2022, 55(6): 1189-1198. |
| [3] | 由玉婉,张雨,孙嘉毅,张蔚. ‘月月粉’月季NAC家族全基因组鉴定及皮刺发育相关成员的筛选[J]. 中国农业科学, 2022, 55(24): 4895-4911. |
| [4] | 胡雪华,刘宁宁,陶慧敏,彭可佳,夏晓剑,胡文海. 低温胁迫对番茄幼苗不同叶龄叶片叶绿素荧光成像特性的影响[J]. 中国农业科学, 2022, 55(24): 4969-4980. |
| [5] | 尤佳玲,李有梅,孙孟豪,谢兆森. ‘黑比诺’葡萄不同叶龄叶片叶绿体内淀粉积累及其相关基因表达差异分析[J]. 中国农业科学, 2022, 55(21): 4265-4278. |
| [6] | 孙保娟,汪瑞,孙光闻,王益奎,李涛,宫超,衡周,游倩,李植良. 转录组及代谢组联合解析茄子果色上位遗传效应[J]. 中国农业科学, 2022, 55(20): 3997-4010. |
| [7] | 刘鑫,张亚红,袁苗,党仕卓,周娟. ‘红地球’葡萄花芽分化过程中的转录组分析[J]. 中国农业科学, 2022, 55(20): 4020-4035. |
| [8] | 崔鹏,赵逸人,姚志鹏,庞林江,陆国权. 低温对甘薯淀粉理化特性及代谢关键基因表达量的影响[J]. 中国农业科学, 2022, 55(19): 3831-3840. |
| [9] | 徐献斌,耿晓月,李慧,孙丽娟,郑焕,陶建敏. 基于转录组分析ABA促进葡萄花青苷积累相关基因[J]. 中国农业科学, 2022, 55(1): 134-151. |
| [10] | 郭永春, 王鹏杰, 金珊, 侯炳豪, 王淑燕, 赵峰, 叶乃兴. 基于WGCNA鉴定茶树响应草甘膦相关的基因共表达模块[J]. 中国农业科学, 2022, 55(1): 152-166. |
| [11] | 陈华枝,范元婵,蒋海宾,王杰,范小雪,祝智威,隆琦,蔡宗兵,郑燕珍,付中民,徐国钧,陈大福,郭睿. 基于纳米孔全长转录组数据完善东方蜜蜂微孢子虫的基因组注释[J]. 中国农业科学, 2021, 54(6): 1288-1300. |
| [12] | 杜宇,祝智威,王杰,王秀娜,蒋海宾,范元婵,范小雪,陈华枝,隆琦,蔡宗兵,熊翠玲,郑燕珍,付中民,陈大福,郭睿. 利用第三代纳米孔长读段测序技术构建和注释蜜蜂球囊菌的全长转录组[J]. 中国农业科学, 2021, 54(4): 864-876. |
| [13] | 肖浏骏,刘蕾蕾,邱小雷,汤亮,曹卫星,朱艳,刘兵. 小麦生长模型对拔节期和孕穗期低温胁迫响应能力的比较[J]. 中国农业科学, 2021, 54(3): 504-521. |
| [14] | 王洁,吴晓宇,杨柳,段巧红,黄家保. 大白菜ACA基因家族的全基因组鉴定与表达分析[J]. 中国农业科学, 2021, 54(22): 4851-4868. |
| [15] | 赵卫松,郭庆港,董丽红,王培培,苏振贺,张晓云,鹿秀云,李社增,马平. 枯草芽孢杆菌NCD-2对棉花根系分泌物L-脯氨酸响应的转录-蛋白质组学联合分析[J]. 中国农业科学, 2021, 54(21): 4585-4600. |
|
||