













中国农业科学 ›› 2021, Vol. 54 ›› Issue (21): 4500-4513.doi: 10.3864/j.issn.0578-1752.2021.21.002
朱芳芳( ),董亚辉(
),董亚辉( ),任真真,王志勇,苏慧慧,库丽霞,陈彦惠(
),任真真,王志勇,苏慧慧,库丽霞,陈彦惠( )
)
收稿日期:2021-04-25
接受日期:2021-06-16
出版日期:2021-11-01
发布日期:2021-11-09
通讯作者:
陈彦惠
作者简介:联系方式:朱芳芳,E-mail: 基金资助:
ZHU FangFang( ),DONG YaHui(
),DONG YaHui( ),REN ZhenZhen,WANG ZhiYong,SU HuiHui,KU LiXia,CHEN YanHui(
),REN ZhenZhen,WANG ZhiYong,SU HuiHui,KU LiXia,CHEN YanHui( )
)
Received:2021-04-25
Accepted:2021-06-16
Online:2021-11-01
Published:2021-11-09
Contact:
YanHui CHEN
摘要:
【目的】干旱是严重影响玉米生长发育进程的一个重要因素。挖掘玉米抗旱相关基因,通过转基因功能验证和转录组分析,解析关键基因在响应干旱胁迫过程中的分子调控机制,为抗旱分子育种和遗传改良提供理论依据。【方法】以玉米自交系B104(WT)为背景材料,利用农杆菌介导方法构建过表达ZmIBH1-1转基因株系(ZmIBH1-1-OE);通过对转基因植株进行草铵膦抗性筛选、标记基因和目的基因PCR检测,以及运用实时荧光定量PCR检测目的基因的表达情况,鉴定阳性植株和株系;以WT和ZmIBH1-1-OE转基因株系为材料,通过干旱处理(20% PEG6000),进行表型鉴定和耐旱生理生化指标测定,验证ZmIBH1-1的抗旱功能;通过对干旱胁迫下玉米4叶期转录组的比较分析,鉴定出差异表达的基因(differentially expressed genes,DEGs);结合DAP-seq(DNA affinity purification sequencing)分析,初步确定ZmIBH1-1蛋白直接调控与抗旱相关的下游靶基因,利用基因组可视化软件IGV(integrative genomics viewer)分析ZmIBH1-1蛋白结合候选靶基因的位置,然后通过Dual-Luciferase试验验证ZmIBH1-1蛋白与靶基因的调控关系。【结果】通过玉米遗传转化获得12个转化事件;T3代中,能同时检测到标记基因Bar和目的基因ZmIBH1-1的植株有458个,实时荧光定量PCR检测结果表明,ZmIBH1-1-OE中ZmIBH1-1的表达量显著高于WT,株系3和株系8表达量最高,将其自交获得T4代转基因株系用于后续试验。在干旱胁迫条件下,ZmIBH1-1-OE株系存活率、叶片相对含水量、叶绿素含量、可溶性蛋白含量及其生理生化指标(超氧化物歧化酶、过氧化物酶、过氧化氢酶活性)均显著高于WT,说明玉米中过量表达ZmIBH1-1赋予玉米更高的耐旱性。转录组分析结果表明,WT与ZmIBH1-1-OE株系在干旱胁迫下有1 214个差异表达基因;Gene Ontology(GO)功能富集分析结果表明,差异表达基因主要涉及生物过程、细胞组分和分子功能,如在生物过程中主要涉及到光合作用、应激响应、脱水响应等;KEGG富集分析表明,差异表达基因主要参与植物激素信号传导、新陈代谢等过程。结合转录组显著差异表达基因和DAP-Seq分析所得到ZmIBH1-1蛋白的靶基因,初步确定ZmIBH1-1蛋白直接调控与抗旱相关的11个候选靶基因,包括2个钙信号相关基因、3个半胱氨酸代谢相关基因、1个bHLH转录因子、1个应激响应蛋白、1个谷胱甘肽转移酶、1个氧化还原过程蛋白和2个乙烯响应因子;基因组可视化结果显示ZmIBH1-1蛋白可以结合靶基因启动子区;随后通过Dual-Luciferase试验进一步表明,ZmIBH1-1蛋白可以直接作用于11个候选靶基因,其中,ZmIBH1-1蛋白可以促进ZmCa-M、ZmSYCO、ZmbHLH54、ZmGlu-r1、ZmCLPB3和ZmP450-99A2的表达,抑制ZmAGD12、ZmCYS、ZmCYSB、ZmERF-107和ZmEIN3的表达。此外,在干旱胁迫下NAC、WRKY、MYB等转录因子在ZmIBH1-1-OE和WT株系中也存在差异表达。【结论】ZmIBH1-1的过表达可以增强玉米苗期的耐旱性;ZmIBH1-1蛋白通过直接调控乙烯信号通路中的ZmERF-107和ZmEIN3的表达提高玉米的耐旱性;ZmIBH1-1蛋白通过直接调控钙信号相关基因ZmCa-M和ZmAGD12增强玉米的耐旱性;ZmIBH1-1蛋白可能通过间接调控NAC、WRKY、MYB等转录因子响应干旱胁迫。
朱芳芳,董亚辉,任真真,王志勇,苏慧慧,库丽霞,陈彦惠. 过表达ZmIBH1-1提高玉米苗期抗旱性[J]. 中国农业科学, 2021, 54(21): 4500-4513.
ZHU FangFang,DONG YaHui,REN ZhenZhen,WANG ZhiYong,SU HuiHui,KU LiXia,CHEN YanHui. Over-expression of ZmIBH1-1 to Improve Drought Resistance in Maize Seedlings[J]. Scientia Agricultura Sinica, 2021, 54(21): 4500-4513.
表1
试验所用引物"
| 引物名称 Primer name | 引物序列 Primer sequence (5′-3′) |
|---|---|
| Bar-F | CATCGAGACAAGCACGGTC |
| Bar-R | AAACCCACGTCATGCCAGTT |
| ZmIBH-F | CAATTACATTTACAATTACCATGGTCATGGCCAGGAAGAGGAC |
| ZmIBH-R | CTCTCTAGACTCACCTAGGATCCTCATTGGGCGGAGAAG |
| qIBH-F | AGGAACCACCGCCAAACC |
| qIBH-R | GCHTCTCCGCAGCAGGAC |
| Tublin-F | CCGCTATCTCCGTCGC |
| Tublin-R | GTTCTTGGATGGCGGTCG |
图4
ZmIBH1-1-OE和野生型株系B104在干旱迫下的表型及各项生理指标变化 A:B104和ZmIBH1-1-OE株系在PEG6000胁迫下的表型;B:B104和ZmIBH1-1-OE株系在PEG6000胁迫下的存活率(n=3,±SD,**P<0.01,n.s不显著);C:B104和ZmIBH1-1-OE株系在PEG6000胁迫下的叶片平均含水量(n=3,±SD,**P<0.01,n.s不显著);D:B104和ZmIBH1-1-OE株系在PEG6000胁迫下的POD、SOD、CAT酶活性测定(n=3,±SD,**P<0.01,n.s不显著);E:B104和ZmIBH1-1-OE株系在PEG6000胁迫下的总叶绿素(Cht)、类胡萝卜素(Car)和可溶性蛋白(SP)含量的变化测定(n=3,±SD,**P<0.01,n.s不显著)"
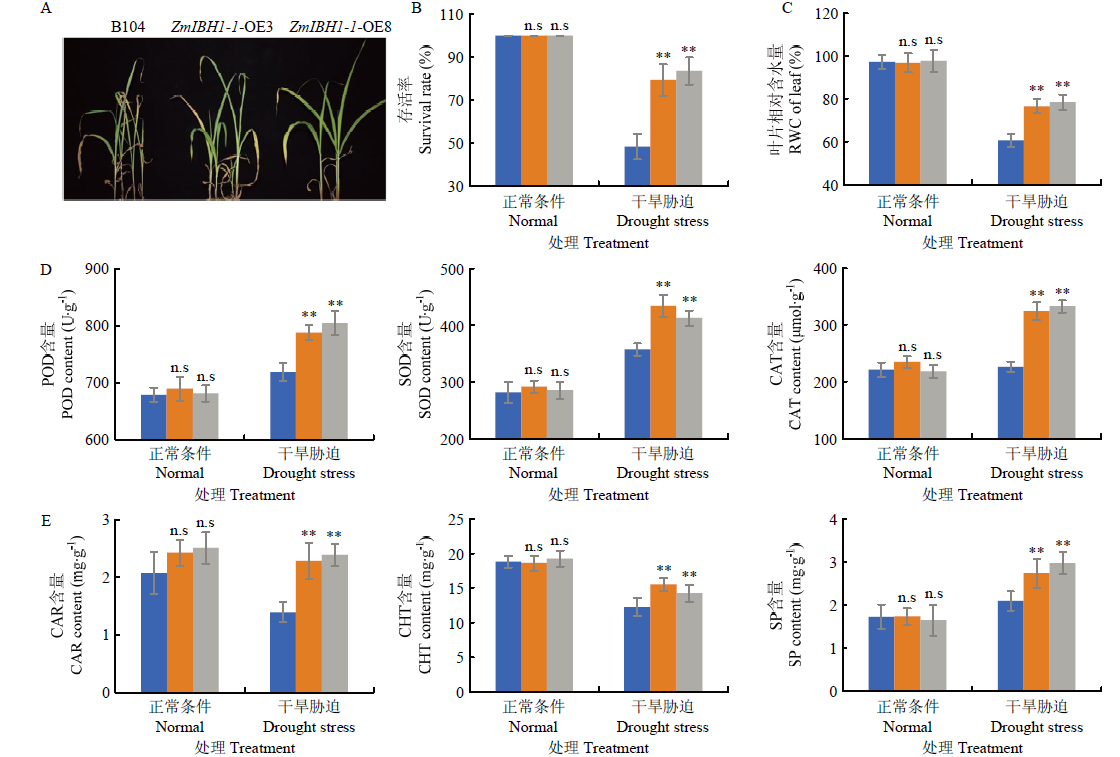
表2
RNA-Seq数据reads数总结"
| 样品 Sample | 正常条件Normal | 干旱胁迫Drought stress | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 原始reads Raw reads | 过滤后的reads Clean reads | 过滤后的 reads所占比 Reads keep rate (%) | 比对上的 reads Mapped reads | 比对上的 reads占比 Mapped reads rate (%) | 原始reads Raw reads | 过滤后的 reads Clean reads | 过滤后的 reads所占比 Reads keep rate (%) | 比对上的reads Mapped reads | 比对上的 reads占比 Mapped reads rate (%) | |
| WT-1 | 5407756 | 5032404 | 93.05 | 4492503 | 89.27 | 3870308 | 3532833 | 91.28 | 3128759 | 88.56 |
| WT-2 | 7232533 | 6586333 | 91.06 | 5987305 | 90.90 | 4119004 | 3813323 | 92.57 | 3482561 | 91.32 |
| OE-1 | 8523635 | 7780152 | 91.27 | 6893250 | 88.60 | 6322179 | 5776743 | 91.37 | 5367210 | 92.91 |
| OE-2 | 7647821 | 6977539 | 91.23 | 6289035 | 90.13 | 6994016 | 6490634 | 92.80 | 5901352 | 90.92 |
| [1] | QU D Y. Food and Agriculture Organization of the United Nations. Viale delle Terme di Caracalla, Rome, Italy. 2016 [June 2021], http://www.fao.org/statistics/databases/en/ . |
| [2] | 代宇佳, 罗晓峰, 周文冠, 陈锋, 帅海威, 杨文钰, 舒凯. 生物和非生物逆境胁迫下的植物系统信号. 植物学报, 2019, 54(2):102-111. |
| DAI Y J, LUO X F, ZHOU W G, CHEN F, SHUAI H W, YANG W Y, SHU K. Plant systemic signaling under biotic and abiotic stresses conditions. Chinese Bulletin of Botany, 2019, 54(2):102-111. (in Chinese) | |
| [3] |
BARTELS D, SUNKAR R. Drought and salt tolerance in plants. Critical Reviews in Plant Sciences, 2005, 24(1):23-58.
doi: 10.1080/07352680590910410 |
| [4] |
MAO H, WANG H, LIU S, LI Z, YANG X, YAN J, LI J, TRAN L, QIN F. A transposable element in a NAC gene is associated with drought tolerance in maize seedlings. Nature Communications, 2015, 6:8326.
doi: 10.1038/ncomms9326 |
| [5] | MAO H, YU L, HAN R, LI Z, LIU H. ZmNAC55, a maize stress-responsive NAC transcription factor, confers drought resistance in transgenic Arabidopsis. Plant Physiology & Biochemistry, 2016, 105:55-66. |
| [6] |
WANG C T, RU J N, LIU Y W, LI M, ZHAO D, YANG J F, FU J D, XU Z S. Maize WRKY transcription factor ZmWRKY106 confers drought and heat tolerance in transgenic plants. International Journal of Molecular Sciences, 2018, 19(10):3046.
doi: 10.3390/ijms19103046 |
| [7] |
WANG C T, RU J N, LIU Y W, YANG J F, MENG L, XU Z S, FU J D. The maize WRKY transcription factor ZmWRKY40 confers drought resistance in transgenic Arabidopsis. International Journal of Molecular Sciences, 2018, 19(9):2580.
doi: 10.3390/ijms19092580 |
| [8] |
YING S, ZHANG D F, JING F, SHI Y S, SONG Y C, WANG T Y, YU L. Cloning and characterization of a maize bZIP transcription factor, ZmbZIP72, confers drought and salt tolerance in transgenic Arabidopsis. Planta, 2012, 235(2):253-266.
doi: 10.1007/s00425-011-1496-7 |
| [9] | 刘彦丹, 英生, 张登峰, 石云素, 宋燕春, 白志川, 王天宇, 黎裕. 玉米逆境胁迫响应基因ZmbZIP71的克隆与表达分析. 植物遗传资源学报, 2011, 12(5):775-781. |
| LIU Y D, YING S, ZHANG D F, SHI Y S, SONG Y C, BAI Z C, WANG T Y, LI Y. Isolation and expression analysis of a stress-responsive gene ZmbZIP71 in maize (Zea mays L.). Journal of Plant Genetic Resources, 2011, 12(5):775-781. (in Chinese) | |
| [10] |
WU J, JIANG Y, LIANG Y, CHEN L, CHEN W, CHENG B. Expression of the maize MYB transcription factor ZmMYB3R enhances drought and salt stress tolerance in transgenic plants. Plant Physiology and Biochemistry, 2019, 137:179-188.
doi: 10.1016/j.plaphy.2019.02.010 |
| [11] |
ZHANG H, XIANG Y, HE N, LIU X, DAI M. Enhanced vitamin C production mediated by an ABA-induced PTP-like nucleotidase improves drought tolerance of Arabidopsis and maize. Molecular Plant, 2020, 13(5):760-776.
doi: 10.1016/j.molp.2020.02.005 |
| [12] |
ZHANG X, MI Y, MAO H, LIU S, QIN F. Genetic variation in ZmTIP1 contributes to root hair elongation and drought tolerance in maize. Plant Biotechnology Journal, 2020, 18(5):1271-1283.
doi: 10.1111/pbi.v18.5 |
| [13] |
DING S, HE F, TANG W, DU H, WANG H. Identification of maize CC-type glutaredoxins that are associated with response to drought stress. Genes, 2019, 10(8):610.
doi: 10.3390/genes10080610 |
| [14] |
LI L, DU Y, HE C, DIETRICH C R, ZHENG J. Maize glossy6 is involved in cuticular wax deposition and drought tolerance. Journal of Experimental Botany, 2019, 70(12):3089-3099.
doi: 10.1093/jxb/erz131 |
| [15] | ZHOU L, ZHOU J, XIONG Y, LIU C, WANG J, WANG G, CAI Y, WU K. Overexpression of a maize plasma membrane intrinsic protein ZmPIP1;1 confers drought and salt tolerance in Arabidopsis. PLoS ONE, 2018, 13(6):e198639. |
| [16] |
WANG H, WANG M, XIA Z. The maize class-I SUMO conjugating enzyme ZmSCE1d is involved in drought stress response. International Journal of Molecular Sciences, 2019, 21(1):29.
doi: 10.3390/ijms21010029 |
| [17] |
LIANG Y, JIANG Y, DU M, LI B, WU J. ZmASR3 from the maize ASR gene family positively regulates drought tolerance in transgenic Arabidopsis. International Journal of Molecular Sciences, 2019, 20(9):2278.
doi: 10.3390/ijms20092278 |
| [18] | FELLER A, MACHEMER K, BRAUN E L, GROTEWOLD E. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant Journal for Cell & Molecular Biology, 2011, 66(1):94-116. |
| [19] |
WANG F, ZHU H, KONG W, PENG R, LIU Q, YAO Q. The antirrhinum AmDEL gene enhances flavonoids accumulation and salt and drought tolerance in transgenic Arabidopsis. Planta, 2016, 244(1):59-73.
doi: 10.1007/s00425-016-2489-3 |
| [20] | WANG F, ZHU H, CHEN D, LI Z, PENG R, YAO Q. A grape bHLH transcription factor gene, VvbHLH1, increases the accumulation of flavonoids and enhances salt and drought tolerance in transgenic Arabidopsis thaliana. Plant Cell Tissue & Organ Culture, 2016, 125(2):387-398. |
| [21] |
DONG Y, WANG C, HAN X, TANG S, LIU S, XIA X, YIN W. A novel bHLH transcription factor PebHLH35 from Populus euphratica confers drought tolerance through regulating stomatal development, photosynthesis and growth in Arabidopsis. Biochemical and Biophysical Research Communications, 2014, 450(1):453-458.
doi: 10.1016/j.bbrc.2014.05.139 |
| [22] |
CUI X, WANG Y X, LIU Z W, WANG W L, LI H, ZHUANG J. Transcriptome-wide identification and expression profile analysis of the bHLH family genes in Camellia sinensis. Functional and Integrative Genomics, 2018, 18(5):489-503.
doi: 10.1007/s10142-018-0608-x |
| [23] |
LIU W, TAI H, LI S, GAO W, ZHAO M, XIE C, LI W X. bHLH122is important for drought and osmotic stress resistance in Arabidopsis and in the repression of ABA catabolism. New Phytologist, 2014, 201(4):1192-1204.
doi: 10.1111/nph.2014.201.issue-4 |
| [24] | SEO J S, JOO J, KIM M J, KIM Y K, NAHM B H, SANG I S, CHEONG J J, LEE J S, KIM J K, YANG D C. OsbHLH148, a basic helix-loop-helix protein, interacts with OsJAZ proteins in a jasmonate signaling pathway leading to drought tolerance in rice. Plant Journal for Cell & Molecular Biology, 2011, 65(6):907-921. |
| [25] |
LI Z, LIU C, ZHANG Y, WANG B, RAN Q, ZHANG J. The bHLH family member ZmPTF1 regulates drought tolerance in maize by promoting root development and ABA synthesis. Journal of Experimental Botany, 2019, 70(19):5471-5486.
doi: 10.1093/jxb/erz307 |
| [26] |
CAO Y, ZENG H, KU L X, REN Z, HAN Y, SU H, DOU D, LIU H, DONG Y, ZHU F. ZmIBH1-1 regulates plant architecture in maize. Journal of Experimental Botany, 2020, 71(10):2943-2955.
doi: 10.1093/jxb/eraa052 |
| [27] |
KENNETH J L, THOMAS D S. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 2002, 25:402-408.
doi: 10.1006/meth.2001.1262 |
| [28] | 韩赞平. 玉米种子活力相关性状QTL定位及相关基因的克隆[D]. 郑州: 河南农业大学, 2014. |
| HAN Z P. QTL mapping of seed vigor related traits and related gene cloning in maize[D]. Zhengzhou: Henan Agricultural University, 2014. (in Chinese) | |
| [29] | 姚晓云, 蓝海军, 邓伟, 陈红萍, 罗晨曦, 况震, 罗宗铭, 王记林, 陈大洲. 水稻淡白叶突变体的叶绿素含量测定及农艺性状比较分析. 江西农业学报, 2020, 32(12):12-15. |
| YAO X Y, LAN H J, DENG W, CHEN H P, LUO C X, KUANG Z, LUO Z M, WANG L J, CHEN D Z. Determination of chlorophyll content and comparative analysis of agronomic traits of pale-white- leaf mutant in rice. Acta Agriculturae Jiangxi, 2020, 32(12):12-15. (in Chinese) | |
| [30] | 焦洁. 考马斯亮蓝G-250染色法测定苜蓿中可溶性蛋白含量. 农业工程技术, 2016, 36(17):33-34. |
| JIAO J. Determination of soluble protein content in Alfalfa by Coomassie brilliant blue G-250 staining. Agricultural Engineering Technology, 2016, 36(17):33-34. (in Chinese) | |
| [31] |
YE J. WEGO: A web tool for plotting GO annotations. Nucleic Acids Research, 2006, 34(Web Server issue):W293-W297.
doi: 10.1093/nar/gkl031 |
| [32] |
PIERIK R, SASIDHARAN R, VOESENEK L. Growth control by ethylene: Adjusting phenotypes to the environment. Journal of Plant Growth Regulation, 2007, 26(2):188-200.
doi: 10.1007/s00344-006-0124-4 |
| [33] | LANAHAN M B. The never ripe mutation blocks ethylene perception in tomato. The Plant Cell, 1994, 6(4):521-530. |
| [34] |
GRBI V, Bleecker A B. Ethylene regulates the timing of leaf senescence in Arabidopsis. The Plant Journal, 1995, 8(4):595-602.
doi: 10.1046/j.1365-313X.1995.8040595.x |
| [35] |
LUO J, MA N, PEI H, CHEN J, LI J, GAO J. A DELLA gene, RhGAI1, is a direct target of EIN3 and mediates ethylene-regulated rose petal cell expansion via repressing the expression of RhCesA2. Journal of Experimental Botany, 2013, 64(16):5075-5084.
doi: 10.1093/jxb/ert296 |
| [36] |
HUA J, MEYEROWITZ E M. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell (Cambridge), 1998, 94(2):261-271.
doi: 10.1016/S0092-8674(00)81425-7 |
| [37] |
GUO H W. Paradigms and paradox in the ethylene signaling pathway and interaction network. Molecular Plant, 2011, 4(4):626-634.
doi: 10.1093/mp/ssr042 |
| [38] | OHME-TAKAGI M, SHINSHI H. Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. The Plant Cell, 1995, 7(2):173-182. |
| [39] |
HATTORI Y, NAGAI K, FURUKAWA S, SONG XJ, KAWANO R, SAKAKIBARA H, WU J, MATSUMOTO T, YOSHIMURA A, KITANO H, MATSUOKA M, MORI H, ASHIKARI M. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature, 2009, 460(7258):1026-1030.
doi: 10.1038/nature08258 |
| [40] | KNIGHT H. Calcium signaling during abiotic stress in plants. International Review of Cytology-a Survey of Cell Biology, 1999, 195:269-324. |
| [41] |
REDDY A, ALI G S, CELESNIK H, DAY I S. Coping with stresses: Roles of calcium- and calcium/calmodulin-regulated gene expression. The Plant Cell, 2011, 23(6):2010-2032.
doi: 10.1105/tpc.111.084988 |
| [42] |
DUBROVINA A S, KISELEV K V, KHRISTENKO V S, ALEYNOVA O A. VaCPK20, a calcium-dependent protein kinase gene of wild grapevine Vitis amurensis Rupr., mediates cold and drought stress tolerance. Journal of Plant Physiology, 2015, 185:1-12.
doi: 10.1016/j.jplph.2015.05.020 |
| [43] |
ZOU J J, LI X D, RATNASEKERA D, WANG C, LIU W X, SONG L F, ZHANG W Z, WU W H. Arabidopsis CALCIUM-DEPENDENT PROTEIN KINASE8 and CATALASE3 function in abscisic acid-mediated signaling and H2O2 homeostasis in stomatal guard cells under drought stress. The Plant Cell, 2015, 27(5):1445-1460.
doi: 10.1105/tpc.15.00144 |
| [44] |
REN Z, ZHANG D, CAO L, ZHANG W, KU L. Functions and regulatory framework of ZmNST3 in maize under lodging and drought stress. Plant Cell and Environment, 2020, 43(9):2272-2286.
doi: 10.1111/pce.v43.9 |
| [45] |
OH JE, KWON Y, KIM JH, NOH H, HONG SW, LEE H. A dual role for MYB60 in stomatal regulation and root growth of Arabidopsis thaliana under drought stress. Plant Molecular Biology, 2011, 77(1/2):91-103.
doi: 10.1007/s11103-011-9796-7 |
| [46] |
NAKABAYASHI R, YONEKURA-SAKAKIBARA K, URANO K, SUZUKI M, YAMADA Y, NISHIZAWA T, MATSUDA F, KOJIMA M, SAKAKIBARA H, SHINOZAKI K. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. The Plant Journal, 2014, 77(3):367-379.
doi: 10.1111/tpj.2014.77.issue-3 |
| [47] |
DAI X Y, XU Y Y, MA Q B, XU W Y, WANG T, XUE Y B, CHONG K. Overexpression of an R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenic Arabidopsis. Plant Physiology, 2007, 143(4):1739-1751.
doi: 10.1104/pp.106.094532 |
| [48] |
HONG S, CHEN S, JIANG J, CHEN F, CHEN Y, GU C, LI P, SONG A, ZHU X, GAO H. Heterologous expression of the chrysanthemum R2R3-MYB Transcription Factor CmMYB2 enhances drought and salinity tolerance, increases hypersensitivity to ABA and delays flowering in Arabidopsis thaliana. Molecular Biotechnology, 2012, 51(2):160-173.
doi: 10.1007/s12033-011-9451-1 |
| [49] |
QIN Y, WANG M, TIAN Y, HE W, LU H, XIA G. Over-expression of TaMYB33 encoding a novel wheat MYB transcription factor increases salt and drought tolerance in Arabidopsis. Molecular Biology Reports, 2012, 39(6):7183-7192.
doi: 10.1007/s11033-012-1550-y |
| [50] | FINATTO T, VIANA V E, WOYANN L G, BUSANELLO C, OLIVEIRA A. Can WRKY transcription factors help plants to overcome environmental challenges? Genetics & Molecular Biology, 2018, 41(3):533-544. |
| [1] | 柴海燕,贾娇,白雪,孟玲敏,张伟,金嵘,吴宏斌,苏前富. 吉林省玉米穗腐病致病镰孢菌的鉴定与部分菌株对杀菌剂的敏感性[J]. 中国农业科学, 2023, 56(1): 64-78. |
| [2] | 古丽旦,刘洋,李方向,成卫宁. 小麦吸浆虫小热激蛋白基因Hsp21.9的克隆及在滞育过程与温度胁迫下的表达特性[J]. 中国农业科学, 2023, 56(1): 79-89. |
| [3] | 赵政鑫,王晓云,田雅洁,王锐,彭青,蔡焕杰. 未来气候条件下秸秆还田和氮肥种类对夏玉米产量及土壤氨挥发的影响[J]. 中国农业科学, 2023, 56(1): 104-117. |
| [4] | 张克坤,陈可钦,李婉平,乔浩蓉,张俊霞,刘凤之,房玉林,王海波. 灌水量对限根栽培‘阳光玫瑰’葡萄果实发育与香气物质积累的影响[J]. 中国农业科学, 2023, 56(1): 129-143. |
| [5] | 胡盛,李阳阳,唐章林,李加纳,曲存民,刘列钊. 干旱胁迫下甘蓝型油菜籽粒含油量和蛋白质含量变化的全基因组关联分析[J]. 中国农业科学, 2023, 56(1): 17-30. |
| [6] | 李周帅,董远,李婷,冯志前,段迎新,杨明羡,徐淑兔,张兴华,薛吉全. 基于杂交种群体的玉米产量及其配合力的全基因组关联分析[J]. 中国农业科学, 2022, 55(9): 1695-1709. |
| [7] | 熊伟仡,徐开未,刘明鹏,肖华,裴丽珍,彭丹丹,陈远学. 不同氮用量对四川春玉米光合特性、氮利用效率及产量的影响[J]. 中国农业科学, 2022, 55(9): 1735-1748. |
| [8] | 李易玲,彭西红,陈平,杜青,任俊波,杨雪丽,雷鹿,雍太文,杨文钰. 减量施氮对套作玉米大豆叶片持绿、光合特性和系统产量的影响[J]. 中国农业科学, 2022, 55(9): 1749-1762. |
| [9] | 邱一蕾,吴帆,张莉,李红亮. 亚致死剂量吡虫啉对中华蜜蜂神经代谢基因表达的影响[J]. 中国农业科学, 2022, 55(8): 1685-1694. |
| [10] | 马小艳,杨瑜,黄冬琳,王朝辉,高亚军,李永刚,吕辉. 小麦化肥减施与不同轮作方式的周年养分平衡及经济效益分析[J]. 中国农业科学, 2022, 55(8): 1589-1603. |
| [11] | 李前,秦裕波,尹彩侠,孔丽丽,王蒙,侯云鹏,孙博,赵胤凯,徐晨,刘志全. 滴灌施肥模式对玉米产量、养分吸收及经济效益的影响[J]. 中国农业科学, 2022, 55(8): 1604-1616. |
| [12] | 张家桦,杨恒山,张玉芹,李从锋,张瑞富,邰继承,周阳晨. 不同滴灌模式对东北春播玉米籽粒淀粉积累及淀粉相关酶活性的影响[J]. 中国农业科学, 2022, 55(7): 1332-1345. |
| [13] | 谭先明,张佳伟,王仲林,谌俊旭,杨峰,杨文钰. 基于PLS的不同水氮条件下带状套作玉米产量预测[J]. 中国农业科学, 2022, 55(6): 1127-1138. |
| [14] | 董桑婕,姜小春,王羚羽,林锐,齐振宇,喻景权,周艳虹. 远红光补光对辣椒幼苗生长和非生物胁迫抗性的影响[J]. 中国农业科学, 2022, 55(6): 1189-1198. |
| [15] | 冯宣军, 潘立腾, 熊浩, 汪青军, 李静威, 张雪梅, 胡尔良, 林海建, 郑洪建, 卢艳丽. 南方地区120份甜、糯玉米自交系重要目标性状和育种潜力分析[J]. 中国农业科学, 2022, 55(5): 856-873. |
|
||