













中国农业科学 ›› 2021, Vol. 54 ›› Issue (19): 4121-4131.doi: 10.3864/j.issn.0578-1752.2021.19.008
收稿日期:2021-02-06
接受日期:2021-02-27
出版日期:2021-10-01
发布日期:2021-10-12
通讯作者:
王建军
作者简介:孟祥坤,E-mail: 基金资助:
MENG XiangKun( ),WU ZhaoLu,YANG XueMei,GUAN DaoJie,WANG JianJun(
),WU ZhaoLu,YANG XueMei,GUAN DaoJie,WANG JianJun( )
)
Received:2021-02-06
Accepted:2021-02-27
Online:2021-10-01
Published:2021-10-12
Contact:
JianJun WANG
摘要:
【目的】克隆二化螟(Chilo suppressalis)P糖蛋白基因(CsPgp)并对其分子特征和表达模式进行分析,明确CsPgp对常用防治杀虫剂氯虫苯甲酰胺和阿维菌素的诱导响应并对其潜在的转录调控机制进行探索。【方法】使用基因克隆技术扩增CsPgp全长基因序列,利用生物信息学技术对CsPgp编码蛋白的分子特征和5′端转录调控区中的转录因子结合位点进行分析。使用荧光定量PCR方法对CsPgp在二化螟不同龄期和不同组织中的表达模式及在杀虫剂氯虫苯甲酰胺和阿维菌素不同剂量处理下的诱导响应进行测定分析。【结果】CsPgp cDNA序列全长4 584 bp,由23个外显子构成,编码1 259个氨基酸,含有两个跨膜区和两个核苷酸结合区,具有ABC转运蛋白家族典型的结构特征,如对底物转运具有重要功能的Walker A、Walker B及D、H、P、Q-Loop等特征序列。CsPgp主要在二化螟幼虫期表达,在3—4龄幼虫中具有最高的表达量,在蛹期和成虫期的表达量较低。组织表达分析表明,CsPgp主要高表达于二化螟的前肠和中肠组织,在后肠、脂肪体、马氏管等其他组织中的表达量较低。相比于对照,使用LC30和LC70剂量氯虫苯甲酰胺分别处理二化螟3龄幼虫12 h和24 h后,CsPgp的表达量未发生显著变化。但在处理36 h后,LC30处理组试虫中CsPgp显著上调表达,而LC70处理组试虫中CsPgp则显著下调表达。使用低剂量0.05 mg·L-1的阿维菌素处理二化螟试虫12 h后,相比于对照,CsPgp显著下调表达,在处理24 h和36 h后CsPgp的表达水平没有发生显著变化,使用0.15 mg·L-1的阿维菌素处理二化螟试虫24 h和36 h后CsPgp被显著诱导上调表达。对CsPgp的5′端转录调控区的序列分析发现,在转录调控区中预测到多个转录因子结合位点,其中包括5个潜在的CncC结合位点。【结论】二化螟CsPgp在重要解毒代谢组织中肠中高表达,并且能够被杀虫剂氯虫苯甲酰胺和阿维菌素诱导表达,表明CsPgp可能参与对氯虫苯甲酰胺和阿维菌素的解毒代谢。CsPgp 5′端转录调控区中含有多个转录因子CncC结合位点,可能对CsPgp的转录表达具有重要调控作用。推测在氯虫苯甲酰胺或阿维菌素胁迫下,CsPgp可能受到转录因子CncC的转录调控并参与对氯虫苯甲酰胺或阿维菌素的解毒代谢。
孟祥坤,吴赵露,杨雪梅,官道杰,王建军. 二化螟P糖蛋白基因的克隆分析及对杀虫剂的诱导响应[J]. 中国农业科学, 2021, 54(19): 4121-4131.
MENG XiangKun,WU ZhaoLu,YANG XueMei,GUAN DaoJie,WANG JianJun. Cloning and Analysis of P-glycoprotein Gene and Its Transcriptional Response to Insecticide in Chilo suppressalis[J]. Scientia Agricultura Sinica, 2021, 54(19): 4121-4131.
表1
用于CsPgp克隆与分析的引物序列"
| 引物名称Primer name | 序列Sequence (5′ to 3′) | 作用Function |
|---|---|---|
| CsPgp F | CAGCTTTGAATCAGGACTTTGC | CsPgp基因片段扩增 Gene fragment amplification of CsPgp |
| CsPgp R | ATCCACTCCGATTGAGGTTGTA | |
| CsPgp 5′R1 | CCATCCCTTCACAAGTGCCATTAT | CsPgp 5′端片段扩增 5′ end fragment amplification of CsPgp |
| CsPgp 5′R2 | CGAACGCCGATTGGTAGAAAAC | |
| CsPgp 3′F1 | GCGTTGGATACGGAAAGCGAAAA | CsPgp 3′端片段扩增 3′ end fragment amplification of CsPgp |
| CsPgp 3′F2 | AGTGCGGGACGCCGACCTTATA | |
| DLCsPgp F | GCAGGATGCACCACTCCTATCA | CsPgp定量分析 Quantitative analysis of CsPgp |
| DLCsPgp R | TGGCCGCTCCGACTATACAGTTA | |
| EF-1α F | TGAACCCCCATACAGCGAATCC | |
| EF-1α R | TCTCCGTGCCAACCAGAAATAGG | |
| CsPgp 5′F | GCACTATTACACTTTTTAATGAG | CsPgp 5′侧翼区DNA序列克隆 5′ lateral DNA sequence cloning of CsPgp |
| CsPgp 5′R | GCGTTTCCGTTCAAATCATAAG |

图1
CsPgp序列特征分析 A:CsPgp核酸序列及其编码的氨基酸序列,深灰色背景标注的为CsPgp跨膜区序列;浅灰色背景标注的为CsPgp核苷酸结合区,其中重要的结构特征使用单下划线标出,斜体氨基酸为ATP结合位点Nucleotide and deduced amino acid sequences of CsPgp. The transmembrane domains are marked by dark grey background, and the nucleotide-binding domains are marked by light grey background. The important structural characteristics in nucleotide-binding domains are underlined, and the italic amino acids indicated as the binding sites of ATP。B:CsPgp基因组结构Genomics structure of CsPgp。Intron:内含子;Exon外显子;UTR:非翻译区Untranslated region"
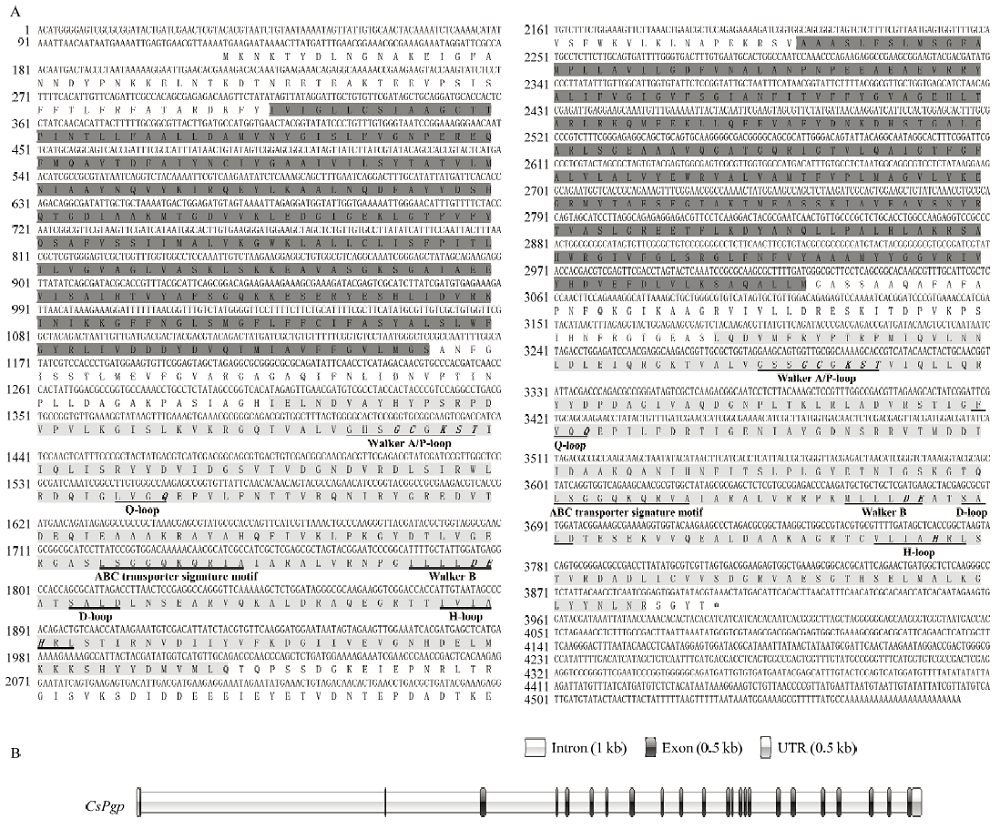

图3
CsPgp在二化螟不同发育时期中的相对表达量 柱上标有不同字母表示CsPgp在不同时期表达量差异显著(P<0.05) Histograms with different letters indicate significant difference of CsPgp expression in different stages (P<0.05)。1—6龄幼虫:1st-6th instar larva;预蛹:Prepupa;1、3、5日蛹:1st, 3rd and 5th day-old pupa;0、12、24、36、48、72小时成虫:0, 12, 24, 36, 48 and 72 hour-old adult"
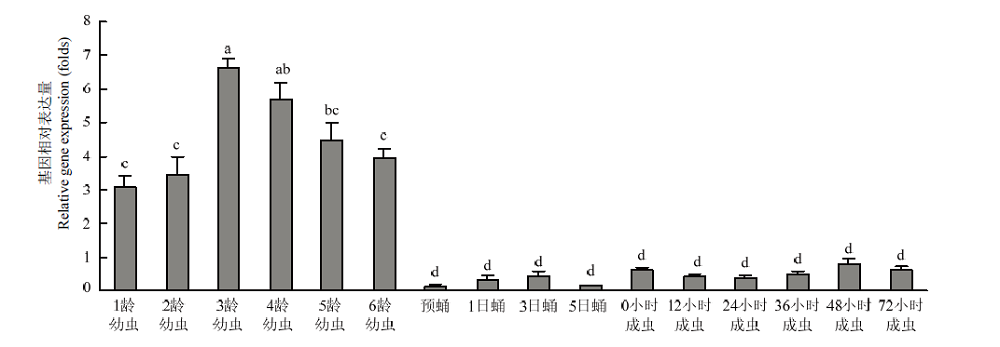
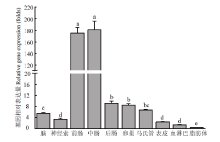
图4
CsPgp在二化螟不同组织中的相对表达量 柱上标有不同字母表示CsPgp在不同组织表达量差异显著(P<0.05) Histograms with different letters indicate significant difference of CsPgp expression in different tissues (P<0.05)。脑:Brain;神经索:Nerve cord;前肠:Foregut;中肠:Midgut;后肠:Hindgut;卵巢:Ovary;马氏管:Malpighian tubule;表皮:Cuticula;血淋巴:Hemolymph;脂肪体:Fat body"
| [1] | 刘万才, 刘振东, 黄冲, 陆明红, 刘杰, 杨清坡. 近10年农作物主要病虫害发生危害情况的统计和分析. 植物保护, 2016, 42(5):1-9. |
| LIU W C, LIU Z D, HUANG C, LU M H, LIU J, YANG Q P. Statistics and analysis of crop yield losses caused by main diseases and insect pests in recent 10 years. Plant Protection, 2016, 42(5):1-9. (in Chinese) | |
| [2] | 全国农业技术推广服务中心. 2017年全国农业有害生物抗药性监测结果及科学用药建议. 中国植保导刊, 2018, 38(4):52-56. |
| National Agricultural Technology Extension Service Center. Monitoring results of pesticide resistance of agricultural pests and suggestions for scientific pesticide use in China in 2017. China Plant Protection, 2018, 38(4):52-56. (in Chinese) | |
| [3] | 全国农业技术推广服务中心. 2018年全国农业有害生物抗药性监测结果及科学用药建议. 中国植保导刊, 2019, 39(3):63-67, 72. |
| National Agricultural Technology Extension Service Center. Monitoring results of pesticide resistance of agricultural pests and suggestions for scientific pesticide use in China in 2018. China Plant Protection, 2019, 39(3):63-67, 72. (in Chinese) | |
| [4] | 全国农业技术推广服务中心. 2019年全国农业有害生物抗药性监测结果及科学用药建议. 中国植保导刊, 2020, 40(3):64-69. |
| National Agricultural Technology Extension Service Center. Monitoring results of pesticide resistance of agricultural pests and suggestions for scientific pesticide use in China in 2019. China Plant Protection, 2020, 40(3):64-69. (in Chinese) | |
| [5] |
WEI Y B, YAN R, ZHOU Q L, QIAO L Y, ZHU G N, CHEN M L. Monitoring and mechanisms of chlorantraniliprole resistance in Chilo suppressalis (Lepidoptera: Crambidae) in China. Journal of Economic Entomology, 2019, 112(3):1348-1353.
doi: 10.1093/jee/toz001 |
| [6] |
LU Y H, WANG G R, ZHONG L Q, ZHANG F C, BAI Q, ZHENG X S, LU Z X. Resistance monitoring of Chilo suppressalis (Walker) (Lepidoptera: Crambidae) to chlorantraniliprole in eight field populations from east and central China. Crop Protection, 2017, 100:196-202.
doi: 10.1016/j.cropro.2017.07.006 |
| [7] |
MAO K K, LI W H, LIAO X, LIU C Y, QIN Y, REN Z J, QIN X Y, WAN H, SHENG F, LI J H. Dynamics of insecticide resistance in different geographical populations of Chilo suppressalis (Lepidoptera: Crambidae) in China 2016-2018. Journal of Economic Entomology, 2019, 112(4):1866-1874.
doi: 10.1093/jee/toz109 |
| [8] |
KALSI M, PALLI S R. Cap n collar transcription factor regulates multiple genes coding for proteins involved in insecticide detoxification in the red flour beetle, Tribolium castaneum. Insect Biochemistry and Molecular Biology, 2017, 90:43-52.
doi: 10.1016/j.ibmb.2017.09.009 |
| [9] |
MENG X K, YANG X M, WU Z L, SHEN Q W, MIAO L J, ZHENG Y, QIAN K, WANG J J. Identification and transcriptional response of ATP-binding cassette transporters to chlorantraniliprole in the rice striped stem borer, Chilo suppressalis. Pest Management Science, 2020, 76(11):3626-3635.
doi: 10.1002/ps.v76.11 |
| [10] |
DERMAUW W, VAN LEEUWEN T. The ABC gene family in arthropods: Comparative genomics and role in insecticide transport and resistance. Insect Biochemistry and Molecular Biology, 2014, 45:89-110.
doi: 10.1016/j.ibmb.2013.11.001 |
| [11] |
SUN Y, XU L, CHEN Q, QIN W J, HUANG S J, JIANG Y, QIN H G. Chlorantraniliprole resistance and its biochemical and new molecular target mechanisms in laboratory and field strains of Chilo suppressalis (Walker). Pest Management Science, 2018, 74(6):1416-1423.
doi: 10.1002/ps.2018.74.issue-6 |
| [12] |
XU L, ZHAO J, SUN Y, XU D J, XU G C, XU X L, ZHANG Y L, HUANG S J, HAN Z J, GU Z Y. Constitutive overexpression of cytochrome P450 monooxygenase genes contributes to chlorantraniliprole resistance in Chilo suppressalis (Walker). Pest Management Science, 2019, 75(3):718-725.
doi: 10.1002/ps.2019.75.issue-3 |
| [13] |
ZHAO J, XU L, SUN Y, SONG P P, HAN Z J. UDP- glycosyltransferase genes in the striped rice stem borer, Chilo suppressalis (Walker), and their contribution to chlorantraniliprole resistance. International Journal of Molecular Sciences, 2019, 20(5):1064.
doi: 10.3390/ijms20051064 |
| [14] | 李波, 韩兰芝, 彭于发. 二化螟人工饲养技术. 应用昆虫学报, 2015, 52(2):498-503. |
| LI B, HAN L Z, PENG Y F. Development of a standardized artificial diet and rearing technique for the striped stem borer, Chilo suppressalis Walker (Lepidoptera: Crambidae). Chinese Journal of Applied Entomology, 2015, 52(2):498-503. (in Chinese) | |
| [15] |
MENG X K, DONG F, QIAN K, MIAO L J, YANG X M, GE H C, WU Z L, WANG J J. Transcriptome analysis reveals global gene expression changes of Chilo suppressalis in response to sublethal dose of chlorantraniliprole. Chemosphere, 2019, 234:648-657.
doi: 10.1016/j.chemosphere.2019.06.129 |
| [16] | 徐红星, 王国荣, 鲁艳辉, 杨亚军, 郑许松, 田俊策, 吕仲贤. 二化螟实时荧光定量PCR内参基因筛选和表达稳定性评价. 中国水稻科学, 2019, 33(1):75-84. |
| XU H X, WANG G R, LU Y H, YANG Y J, ZHENG X S, TIAN J C, LÜ Z X. Screening reference genes and evaluating of their expression stability for qRT-PCR normalization in Chilo suppressalis (Lepidoptera: Pyralididae). Chinese Journal of Rice Science, 2019, 33(1):75-84. (in Chinese) | |
| [17] | XU J, LU M X, CUI Y D, DU Y Z. Selection and evaluation of reference genes for expression analysis using qRT-PCR in Chilo suppressalis (Lepidoptera: Pyralidae). Journal of Economic Entomology, 2017, 110(2):683-691. |
| [18] |
DERMAUW W, ILIAS A, RIGA M, TSAGKARAKOU A, GRBIC M, TIRRY L, VAN LEEUWEN T, VONTAS J. The cys-loop ligand-gated ion channel gene family of Tetranychus urticae: Implications for acaricide toxicology and a novel mutation associated with abamectin resistance. Insect Biochemistry and Molecular Biology, 2012, 42(7):455-465.
doi: 10.1016/j.ibmb.2012.03.002 |
| [19] |
LIAO C Y, XIA W K, FENG Y C, LI G, LIU H, DOU W, WANG J J. Characterization and functional analysis of a novel glutathione S-transferase gene potentially associated with the abamectin resistance in Panonychus citri (McGregor). Pesticide Biochemistry and Physiology, 2016, 132:72-80.
doi: 10.1016/j.pestbp.2015.11.002 |
| [20] |
RIGA M, TSAKIRELI D, ILIAS A, MOROU E, MYRIDAKIS A, STEPHANOU E G, NAUEN R, DERMAUW W, VAN LEEUWEN T, PAINE M, VONTAS J. Abamectin is metabolized by CYP392A16, a cytochrome P450 associated with high levels of acaricide resistance in Tetranychus urticae. Insect Biochemistry and Molecular Biology, 2014, 46:43-53.
doi: 10.1016/j.ibmb.2014.01.006 |
| [21] |
WANG X L, PUINEAN A M, O’REILLY A O, WILLIAMSON M S, SMELT C L C, MILLAR N S, WU Y D. Mutations on M3 helix of Plutella xylostella glutamate-gated chloride channel confer unequal resistance to abamectin by two different mechanisms. Insect Biochemistry and Molecular Biology, 2017, 86:50-57.
doi: 10.1016/j.ibmb.2017.05.006 |
| [22] | YIN Q, QIAN L, SONG P P, JIAN T Y, HAN Z J. Molecular mechanisms conferring asymmetrical cross-resistance between tebufenozide and abamectin in Plutella xylostella. Journal of Asia-Pacific Entomology, 2019, 22(1):189-193. |
| [23] |
LUO L, SUN Y J, WU Y J. Abamectin resistance in Drosophila is related to increased expression of P-glycoprotein via the dEGFR and dAkt pathways. Insect Biochemistry and Molecular Biology, 2013, 43(8):627-634.
doi: 10.1016/j.ibmb.2013.04.006 |
| [24] |
XIANG M, ZHANG L, LU Y, TANG Q L, LIANG P, SHI X Y, SONG D L, GAO X W. A P-glycoprotein gene serves as a component of the protective mechanisms against 2-tridecanone and abamectin in Helicoverpa armigera. Gene, 2017, 627:63-71.
doi: 10.1016/j.gene.2017.06.010 |
| [25] |
TIAN L X, YANG J Q, HOU W J, XU B Y, XIE W, WANG S L, ZHANG Y J, ZHOU X G, WU Q J. Molecular cloning and characterization of a P-glycoprotein from the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). International Journal of Molecular Sciences, 2013, 14(11):22891-22905.
doi: 10.3390/ijms141122891 |
| [26] |
ENDERS L S, RAULT L C, HENG-MOSS T M, SIEGFRIED B D, MILLER N J. Transcriptional responses of soybean aphids to sublethal insecticide exposure. Insect Biochemistry and Molecular Biology, 2020, 118:103285.
doi: 10.1016/j.ibmb.2019.103285 |
| [27] |
TERRIERE L C. Induction of detoxication enzymes in insects. Annual Review of Entomology, 1984, 29:71-88.
doi: 10.1146/ento.1984.29.issue-1 |
| [28] |
HE C, LIANG J J, LIU S N, WANG S L, WU Q J, XIE W, ZHANG Y J. Changes in the expression of four ABC transporter genes in response to imidacloprid in Bemisia tabaci Q (Hemiptera: Aleyrodidae). Pesticide Biochemistry and Physiology, 2019, 153:136-143.
doi: 10.1016/j.pestbp.2018.11.014 |
| [29] |
JIN M H, LIAO C Y, CHAKRABARTY S, ZHENG W G, WU K M, XIAO Y T. Transcriptional response of ATP-binding cassette (ABC) transporters to insecticides in the cotton bollworm, Helicoverpa armigera. Pesticide Biochemistry and Physiology, 2019, 154:46-59.
doi: 10.1016/j.pestbp.2018.12.007 |
| [30] | MERZENDORFER H. ABC transporters and their role in protecting insects from pesticides and their metabolites//COHEN E. Target Receptors in the Control of Insect Pests: Part II. 2014, 46:1-72. |
| [31] |
SUN H, PU J, CHEN F, WANG J D, HAN Z J. Multiple ATP-binding cassette transporters are involved in insecticide resistance in the small brown planthopper, Laodelphax striatellus. Insect Molecular Biology, 2017, 26(3):343-355.
doi: 10.1111/imb.2017.26.issue-3 |
| [32] |
XU Z F, SHI L, PENG J F, SHEN G M, WEI P, WU Q, HE L. Analysis of the relationship between P-glycoprotein and abamectin resistance in Tetranychus cinnabarinus (Boisduval). Pesticide Biochemistry and Physiology, 2016, 129:75-82.
doi: 10.1016/j.pestbp.2015.10.021 |
| [33] |
ZUO Y Y, HUANG J L, WANG J, FENG Y, HAN T T, WU Y D, YANG Y H. Knockout of a P-glycoprotein gene increases susceptibility to abamectin and emamectin benzoate in Spodoptera exigua. Insect Molecular Biology, 2018, 27(1):36-45.
doi: 10.1111/imb.12338 pmid: 28753233 |
| [34] |
BAKSHI M, OELMÜLLER R. WRKY transcription factors: Jack of many trades in plants. Plant Signaling and Behavior, 2014, 9(2):e27700.
doi: 10.4161/psb.27700 |
| [35] |
HU B, HU S Z, HUANG H, WEI Q, REN M M, HUANG S F, TIAN X R, SU J Y. Insecticides induce the co-expression of glutathione S-transferases through ROS/CncC pathway in Spodoptera exigua. Pesticide Biochemistry and Physiology, 2019, 155:58-71.
doi: 10.1016/j.pestbp.2019.01.008 |
| [36] |
WILDING C S. Regulating resistance: CncC:Maf, antioxidant response elements and the overexpression of detoxification genes in insecticide resistance. Current Opinion in Insect Science, 2018, 27:89-96.
doi: 10.1016/j.cois.2018.04.006 |
| [37] |
CHEN L, ZHANG T T, GE M Y, LIU Y H, XING Y P, LIU L, LI F L, CHENG L G. The Nrf2-Keap1 pathway: A secret weapon against pesticide persecution in Drosophila Kc cells. Pesticide Biochemistry and Physiology, 2020, 164:47-57.
doi: 10.1016/j.pestbp.2019.12.008 |
| [38] |
CHENG X Y, HU J H, LI J X, CHEN J, WANG H, MAO T T, XUE B, LI B. The silk gland damage and the transcriptional response to detoxifying enzymes-related genes of Bombyx mori under phoxim exposure. Chemosphere, 2018, 209:964-971.
doi: 10.1016/j.chemosphere.2018.06.167 |
| [39] |
MAO T T, LI F C, FANG Y L, WANG H, CHEN J, LI M X, LU Z T, QU J W, LI J X, HU J H, CHENG X Y, NI M, LI B. Effects of chlorantraniliprole exposure on detoxification enzyme activities and detoxification-related gene expression in the fat body of the silkworm, Bombyx mori. Ecotoxicology and Environmental Safety, 2019, 176:58-63.
doi: 10.1016/j.ecoenv.2019.03.074 |
| [40] | SHI L, SHI Y, LIU M F, ZHANG Y, LIAO X L. Transcription factor CncC potentially regulates the expression of multiple detoxification genes that mediate indoxacarb resistance in Spodoptera litura. Insect Science, 2021, https://doi.org/10.1111/1744-7917.12860. |
| [1] | 张洁,姜长岳,王跃进. 中国野生毛葡萄转录因子VqWRKY6与VqbZIP1互作调控抗白粉病功能分析[J]. 中国农业科学, 2022, 55(23): 4626-4639. |
| [2] | 李正刚,汤亚飞,佘小漫,于琳,蓝国兵,何自福. 侵染萝卜的油菜花叶病毒广东分离物分子特征及其致病性分析[J]. 中国农业科学, 2022, 55(14): 2752-2761. |
| [3] | 杜宇,王永,孟庆勇,朱江江,林亚秋. 干扰山羊KLF12促进皮下脂肪细胞分化[J]. 中国农业科学, 2022, 55(1): 184-196. |
| [4] | 王萍,郑晨飞,王娇,胡璋健,邵淑君,师恺. 番茄转录因子SlNAC29在调控植株衰老中的作用及机理[J]. 中国农业科学, 2021, 54(24): 5266-5276. |
| [5] | 张丽,汤亚飞,李正刚,于琳,蓝国兵,佘小漫,何自福. 侵染广东省葫芦科作物的中国南瓜曲叶病毒的分子特征[J]. 中国农业科学, 2021, 54(19): 4097-4109. |
| [6] | 杜星,曾强,刘禄,李琦琦,杨柳,潘增祥,李齐发. 二花脸猪linc-NORFA核心启动子鉴定与转录调控分析[J]. 中国农业科学, 2021, 54(15): 3331-3342. |
| [7] | 李正刚,农媛,汤亚飞,佘小漫,于琳,蓝国兵,邓铭光,何自福. 侵染广东连州葫芦的黄瓜绿斑驳花叶病毒的分子特征 及致病性分析[J]. 中国农业科学, 2020, 53(5): 955-964. |
| [8] | 王峰,王秀杰,赵胜男,闫家榕,卜鑫,张颖,刘玉凤,许涛,齐明芳,齐红岩,李天来. 光对园艺植物花青素生物合成的调控作用[J]. 中国农业科学, 2020, 53(23): 4904-4917. |
| [9] | 何雨娟,鞠迪,王悦,杨雪清,王小奇. 水稻蛋白酶抑制剂基因OsLTPL164和OsLTPL151的组成型及诱导型表达模式[J]. 中国农业科学, 2018, 51(12): 2311-2321. |
| [10] | 鲁艳辉,高广春,郑许松,吕仲贤. 诱集植物香根草对二化螟幼虫致死的作用机制[J]. 中国农业科学, 2017, 50(3): 486-495. |
| [11] | 韩立强,王月影,王林枫,朱河水,钟凯,褚贝贝,杨国宇. 奶牛SREBP1蛋白在乳腺上皮细胞的表达定位及对SCD1基因启动子的转录调控[J]. 中国农业科学, 2016, 49(24): 4797-4805. |
| [12] | 王亚娴,杨藩,王华岩. Sall4表达调控及其启动子核心调控区的筛选[J]. 中国农业科学, 2016, 49(1): 176-185. |
| [13] | 徐汇洋,许榜丰,陈艳,隋金钰,杨焕良,尹航,杨大为,乔传玲,陈化兰. 一株H1N1猪流感病毒的进化分析与分子特征[J]. 中国农业科学, 2015, 48(15): 3071-3078. |
| [14] | 刘辉,李德军,邓治. 植物应答低温胁迫的转录调控网络研究进展[J]. 中国农业科学, 2014, 47(18): 3523-3533. |
| [15] | 郑本川, 张锦芳, 李浩杰, 柴靓, 崔成, 蒋俊, 蒲晓斌, 牛应泽, 蒋梁材. 甘蓝型油菜开花调控转录因子CONSTANS的表达分析[J]. 中国农业科学, 2013, 46(12): 2592-2598. |
|
||