













中国农业科学 ›› 2020, Vol. 53 ›› Issue (22): 4584-4600.doi: 10.3864/j.issn.0578-1752.2020.22.006
唐科志,周常勇
收稿日期:2020-01-19
接受日期:2020-03-27
出版日期:2020-11-16
发布日期:2020-11-28
通讯作者:
周常勇
基金资助:TANG KeZhi,ZHOU ChangYong
Received:2020-01-19
Accepted:2020-03-27
Online:2020-11-16
Published:2020-11-28
Contact:
ChangYong ZHOU
摘要: 【目的】 明确红橘(Citrus reticulata Blanco, cv. Hongjv)接种褐斑病致病菌——链格孢菌橘致病型(Alternaria alternata tangerine pathotype)后基因种类和表达量在转录水平的变化规律,确定红橘响应该致病型侵染的关键基因。【方法】 采用链格孢菌橘致病型接种红橘离体叶片,28 h后选取感病叶片和未接种叶片提取RNA,进行转录组高通量测序,然后利用生物信息学分析,以甜橙基因组为参考,以|log2 fold change|≥1,q-value≤0.01为阈值选取感病和健康红橘叶片转录组的差异表达基因,应用GO数据库对差异表达基因(differentially expressed gene,DEG)进行功能分类,KEGG分析代谢途径,MapMan软件分析生物胁迫信号通路相关基因的表达变化。采用qRT-PCR方法对测序结果进行验证。【结果】 红橘接种链格孢菌橘致病型28 h后产生大量与胁迫相关的差异表达基因,获得上调差异基因5 173个,下调差异基因6 555个。GO功能分类显示差异基因主要与蛋白结合、膜、氧化还原过程等相关。通过KEGG富集和MapMan软件分析发现,红橘在受链格孢菌橘致病型胁迫的过程中基础代谢被严重破坏。乙烯、水杨酸和生长素等寄主防御相关的植物激素信号转导途径多个基因表达出现差异,其中乙烯起主导作用,乙烯受体ETR 3个成员被不同程度激活,下游激酶和乙烯响应因子均上调,生长素大部分关键信号基因、绝大部分生长素响应因子ARF和水杨酸合成途径的基因均下调表达。同时,黄酮醇、花青素、萜类化合物和生物碱合成相关基因受该菌诱导显著变化,萜类合成中大部分基因下调,而黄酮类合成相关上调基因数量和表达趋势均强于表达下调基因,有抗虫和抑菌作用的硫代葡萄糖苷基因呈上调趋势。进一步研究发现,大量参与抗逆过程的转录因子如WRKY、bZIP、ERF、MYB、NAC被诱导激活,其中大部分WRKY和bZIP转录因子受该菌正向调控,超过50%的ERF家族基因表达上调;在转录因子调控下,PTI及ETI响应基因如受体激酶、R蛋白、NBS抗病蛋白等大量表达,多个PR家族抗菌蛋白基因上调表达,22个抗氧化保护酶系统POD成员基因受到活性氧信号激发大量表达。以上结果表明链格孢菌橘致病型侵染对寄主内部生理状态产生显著影响。选取了19个与植物抗病相关基因进行qRT-PCR分析,其基因表达趋势与测序数据一致。【结论】 获得了红橘响应链格孢菌橘致病型侵染的差异表达基因及显著上调表达基因,其主要富集于代谢过程、应激反应及转录调控等条目中,这些基因的相互协同调控是红橘对该致病型产生防御反应的重要机制。
唐科志,周常勇. 红橘响应褐斑病菌侵染的转录组学分析[J]. 中国农业科学, 2020, 53(22): 4584-4600.
TANG KeZhi,ZHOU ChangYong. Transcriptome Analysis of Citrus reticulata Blanco, cv. Hongjv Infected with Alternaria alternata Tangerine Pathotype[J]. Scientia Agricultura Sinica, 2020, 53(22): 4584-4600.
表1
qRT-PCR验证基因选择及引物设计"
| 基因ID Gene ID | 引物序列 Primer sequence (5′-3′) | 产物长度 Length of product (bp) | |
|---|---|---|---|
| Cs4g12760 | F: TTCGTCTTGCTCTTCGGATAA | R: GCACTCCAACGGAATCTCTAA | 202 |
| Cs2g09310 | F: GGTGTCATTCCTCCTCCTACC | R: GCAGTTCCCTCGCCTATTCT | 198 |
| Cs5g15470 | F: CAGCCTTGTCGGTATGAGAA | R: CAACACCCAATCTTCCTTGAG | 151 |
| Cs5g21860 | F: GCTCTTGTGGGCATTCTTGC | R: CTCTCGTGTAGAAGCTCTTGCC | 142 |
| Cs7g20700 | F: ACGGTTCAGGCTCATTTCAG | R: TGGGATTTGGCATCATCAAT | 185 |
| Cs3g10430 | F: TGGAGACGTAGAGGCTGTCAA | R: CATACCAATATTTTGAGCATTTT | 121 |
| Cs6g07410 | F: GGAGAGTGGTGGAATGCTGAT | R: CACTTCGAGCGTGTAGGTTTG | 150 |
| Cs6g20850 | F: CCAGCAGGCATGAGAAATTA | R: TGACCATCGTGGGAACAGTA | 229 |
| Cs1g01180 | F: TGCAGTAGAAGTTGATGGTGATG | R: AATGAGCCGTTAGCGACAAG | 165 |
| Cs1g04680 | F: AGCTGCAAGGGTTTGGTTAG | R: GAATTTGCGTTTGGTGATGA | 150 |
| Cs8g13680 | F: GCCTATGCTTGCTGTTGTTTC | R: GAAGGCAGTCCATCCATACTTC | 194 |
| orange1.1t03118 | F: CAAGCTCTCCAGGCAAGAGT | R: GGTCCACGGCCATAGTAGAA | 247 |
| orange1.1t03618 | F: CGGGATGAACATTTGGTTTA | R: CTAGCCTTCTGATCTTGACACA | 184 |
| orange1.1t05311 | F: GCTGGGATATAACTCCTTCTCA | R: TTCCGCTAAACCAATCACTT | 172 |
| Cs2g06120 | F: GCACAAGGAAATGGGTTTGT | R: GAAACACGCTGGGATCACTT | 229 |
| Cs6g07400 | F: ACATGGCTGCAAGAGCATAC | R: CCATTGAGGTCCACCACTTA | 197 |
| orange1.1t00214 | F: ACGCTCTGTCCCTCAACAAG | R: CCGCTACTGCCTCCTGTATC | 171 |
| orange1.1t02319 | F: GGGATCTACTGCCGACACTC | R: CGACGACGACCTTTGATCTT | 245 |
| orange1.1t03603 | F: GGACAATGCTGATCCGAAAG | R: TCAACCAAGCCTCCTGAAAC | 203 |
| CitActin | F: CATCCCTCAGCACCTTCC | R: CCAACCTTAGCACTTCTCC | 191 |
表2
比对参考基因组结果统计"
| 样本 Sample | 总序列数 Total reads | 比对上序列比例 Total mapped reads (%) | 比对上唯一位置序列比例 Unique mapped reads (%) | 双端比对上序列比例 Reads mapped in paired (%) |
|---|---|---|---|---|
| C0_1 | 47713764 | 90.30 | 86.99 | 84.18 |
| C0_2 | 54696950 | 88.96 | 85.64 | 82.20 |
| C0_3 | 52284118 | 89.93 | 86.59 | 83.36 |
| ZC_1 | 55857454 | 75.22 | 71.84 | 69.94 |
| ZC_2 | 53372222 | 74.18 | 70.98 | 68.37 |
| ZC_3 | 57202046 | 74.31 | 70.99 | 68.87 |

图3
差异表达基因WEGO分类 横坐标表示GO的3个方面;左侧纵坐标表示基因数目的百分比;右侧纵坐标表示对应GO term的基因数X axis: Three aspects of GO; The left ordinate: The percentage of the number of genes; The right ordinate: The corresponding GO term gene numberBP1:生物黏附Biological adhesion;BP2:生物调节Biological regulation;BP3:碳利用Carbon utilization;BP4:细胞增殖Cell proliferation;BP5:细胞成分组成或发生Cellular component organization or biogenesis;BP6:细胞过程Cellular process;BP7:解毒Detoxification;BP8:发展过程Developmental process;BP9:增长Growth;BP10:免疫系统过程Immune system process;BP11:定位Localization;BP12:运动Locomotion;BP13:代谢过程Metabolic process;BP14:多生物过程Multi-organism process;BP15:多细胞生物过程Multicellular organismal process;BP16:生物过程负调控Negative regulation of biological process;BP17:氮利用Nitrogen utilization;BP18:生物过程正调控Positive regulation of biological process;BP19:生物过程调节Regulation of biological process;BP20:繁殖Reproduction;BP21:生殖过程Reproductive process;BP22:刺激反应Response to stimulus;BP23:节律过程Rhythmic process;BP24:信号Signaling;CC1:细胞Cell;CC2:细胞部分Cell part;CC3:细胞外区域Extracellular region;CC4:细胞外区域部分Extracellular region part;CC5:大分子复合物Macromolecular complex;CC6:膜Membrane;CC7:膜部分Membrane part;CC8:膜包围腔Membrane-enclosed lumen;CC9:细胞器Organelle;CC10:细胞器部分Organelle part;CC11:超分子复合物Supramolecular complex;MF1:抗氧化活性Antioxidant activity;MF2:绑定Binding;MF3:催化活性Catalytic activity;MF4:电子载体活性Electron carrier activity;MF5:功能分子调控Molecular function regulator;MF6:分子传感器活性Molecular transducer activity;MF7:核酸结合转录因子活性Nucleic acid binding transcription factor activity;MF8:营养库活性Nutrient reservoir activity;MF9:信号传感器活性Signal transducer activity;MF10:结构分子活性Structural molecule activity;MF11:转录因子活性、蛋白质结合Transcription factor activity, protein binding;MF12:转运活性Transporter activity"
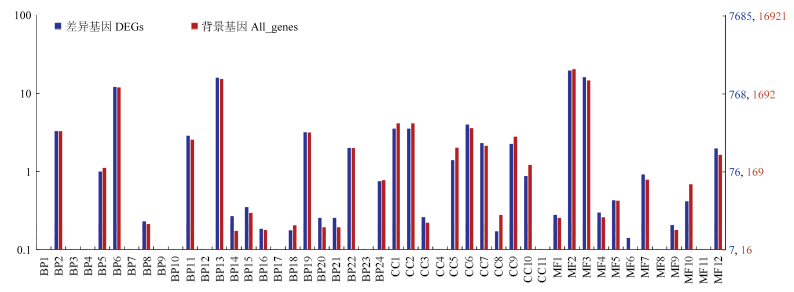

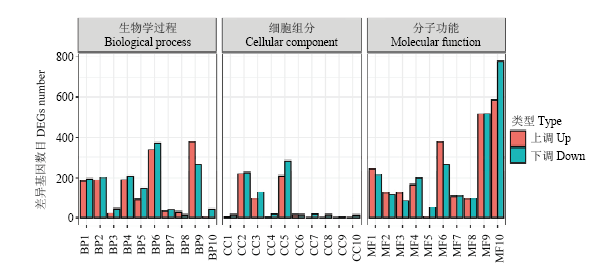
图4
差异表达基因GO分类 BP1:跨膜运输Transmembrane transport;BP2:细胞氧化还原内稳态Cell redox homeostasis;BP3:转录调节、DNA模板化Regulation of transcription, DNA-templated;BP4:碳水化合物代谢过程Carbohydrate metabolic process;BP5:氧化还原过程Oxidation-reduction process;BP6:脂质代谢过程Lipid metabolic process;BP7:花粉识别Recognition of pollen;BP8:蛋白质磷酸化Protein phosphorylation;BP9:基于微管运动Microtubule-based movement;BP10:代谢过程Metabolic process;CC1:叶绿体Chloroplast;CC2:核Nucleus;CC3:微管Microtubule;CC4:膜Membrane;CC5:细胞外区域Extracellular region;CC6:光系统II Photosystem II;CC7:光系统II氧进化复合体Photosystem II oxygen evolving complex;CC8:光系统I反应中心Photosystem I reaction center;CC9:膜的外部成分Extrinsic component of membrane;CC10:膜的组成部分Integral component of membrane;MF1:催化活性Catalytic activity;MF2:DNA结合转录因子活性DNA-binding transcription factor activity;MF3:氧化还原酶活性Oxidoreductase activity;MF4:微管结合Microtubule binding;MF5:蛋白激酶活性Protein kinase activity;MF6:铁离子结合Iron ion binding;MF7:氧化还原酶活性,作用于配对供体,结合或还原分子Oxidoreductase activity, acting on paired donors, with incorporation or reduction of molecular;MF8:ATP结合ATP binding;MF9:蛋白结合Protein binding;MF10:血红素结合Heme binding"
| [1] |
KOHMOTO K, SCHEFFER R P, WHITESIDE J O . Host-selective toxins from Alternaria citri. Phytopathology, 1979,69(6):667-671.
doi: 10.1094/Phyto-69-667 |
| [2] |
NISHIMURA S, KOHMOTO K . Host-specific toxins and chemical structures from Alternaria species. Annual Review of Phytopathology, 1983,21:87-116.
doi: 10.1146/annurev.py.21.090183.000511 pmid: 25946338 |
| [3] | 李红叶, 梅秀凤, 符雨诗, 黄峰, 陈再廖, 杨小平, 王一光, 黄海华 . 柑橘链格孢褐斑病的发生危害风险和治理对策. 果树学报, 2015,32(5):969-976. |
| LI H Y, MEI X F, FU Y S, HUANG F, CHEN Z L, YANG X P, WANG Y G, HUANG H H . Alternaria brown spot of citrus: The risk and management strategy. Journal of Fruit Science, 2015,32(5):969-976. (in Chinese) | |
| [4] | COBB N . Letters on the diseases of plants- Alternaria of the citrus tribe. Agricultural Gazette of New South Wales, 1903,14:955-986. |
| [5] |
WANG X F, LI Z A, TANG K Z, ZHOU C Y, YI L . First report of Alternaria brown spot of citrus caused by Alternaria alternata in Yunnan Province, China. Plant Disease, 2010,94(3):375.
doi: 10.1094/PDIS-94-3-0375B pmid: 30754225 |
| [6] | 陈昌胜, 黄峰, 程兰, 冯春刚, 黄涛江, 李红叶 . 红橘褐斑病病原鉴定. 植物病理学报, 2011,41(5):449-455. |
| CHEN C S, HUANG F, CHENG L, FENG C G, HUANG T J, LI H Y . Identification of the pathogenic fungus causing brown spot on tangerine ( Citrus reticulate cv. Hongjv). Acta Phytopathologica Sinica, 2011,41(5):449-455. (in Chinese) | |
| [7] | 黄峰, 朱丽, 侯欣, 李红叶 . 瓯柑褐斑病病原鉴定. 浙江农业科学, 2012(9):1281-1282. |
| HUANG F, ZHU L, HOU X, LI H Y . Identification of the pathogenic fungus causing brown spot onCitrus suavissima Hort. ex Tanaka. Zhejiang Agricultural Sciences, 2012(9):1281-1282. (in Chinese) | |
| [8] | 赵圆, 王玲杰, 王雪峰, 唐科志, 周常勇, 李太盛 . 杂柑褐斑病的病原鉴定. 果树学报, 2014,31(2):292-295. |
| ZHAO Y, WANG L J, WANG X F, TANG K Z, ZHOU C Y, LI T S . Identification of the pathogenic fungus causing brown spot in two tangerine hybrid varieties. Journal of Fruit Science, 2014,31(2):292-295. (in Chinese) | |
| [9] | CHUNG K R . Mitogen-activated protein kinase signaling pathways of the tangerine pathotype of Alternaria alternate. MAP Kinase, 2013,2(4):16-23. |
| [10] |
YANG S L, YU P L, CHUNG K R . The glutathione peroxidase- mediated reactive oxygen species resistance, fungicide sensitivity and cell wall construction in the citrus fungal pathogen Alternaria alternate. Environmental Microbiology, 2016,18(3):923-935.
doi: 10.1111/1462-2920.13125 pmid: 26567914 |
| [11] | 唐飞艳, 孔向雯, 吕韦玮, 唐科志 . 柑橘褐斑病菌AaSIP2基因生物学功能初步研究. 园艺学报, 2018,45(12):2358-2370. |
| TANG F Y, KONG X W, LÜ W W, TANG K Z . Preliminary studies on the function of AaSIP2 gene in the tangerine pathotype of Alternaria alternata. Acta Horticulturae Sinica, 2018,45(12):2358-2370. (in Chinese) | |
| [12] | MA H J, ZHANG B, GAI Y P, SUN X P, CHUNG K R , LI H Y. Cell-wall-degrading enzymes required for virulence in the host selective toxin-producing necrotroph Alternaria alternata of citrus. Frontiers in Microbiology, 2019, 10: Article 2514. |
| [13] |
WANG M S, SUN X P, YU D L, XU J P, CHUNG K R, LI H Y . Genomic and transcriptomic analyses of the tangerine pathotype of Alternaria alternata in response to oxidative stress. Scientific Reports, 2016,6:32437.
doi: 10.1038/srep32437 pmid: 27582273 |
| [14] | 王明爽 . 柑橘褐斑病菌比较基因组和转录组分析及柑橘绿霉病菌产孢中心调控途径和高渗甘油途径的功能基因研究[D]. 杭州: 浙江大学, 2016. |
| WANG M S . Comparative genome and transcriptome analysis of the tangerine pathotype of Alternaria alternate and functional analysis of genes involved in the central regulatory pathway of conidiation and the high osmolarity glycerol pathway in Penicillium digitatum[D]. Hangzhou: Zhejiang University, 2016. (in Chinese) | |
| [15] |
WU J, ZHANG Y L, ZHANG H Q, HUANG H, FOLTA K M, LU J . Whole genome wide expression profiles of Vitis amurensis grape responding to downy mildew by using Solexa sequencing technology. BMC Plant Biology, 2010,10:234.
doi: 10.1186/1471-2229-10-234 pmid: 21029438 |
| [16] |
XU L, ZHU L F, TU L L, LIU L L, YUAN D J, LIN L, LONG L, ZHANG X L . Lignin metabolism has a central role in the resistance of cotton to the wilt fungus Verticillium dahliae as revealed by RNA-Seq-dependent transcriptional analysis and histochemistry. Journal of Experimental Botany, 2011,62(15):5607-5621.
doi: 10.1093/jxb/err245 |
| [17] | WARD J A, WEBER C A . Comparative RNA-seq for the investigation of resistance to Phytophthora root rot in the red raspberry ‘Latham’. Acta Horticulturae, 2012,946(946):67-72. |
| [18] | WANG Y S, ZHOU L J, YU X Y, STOVER E, LUO F , DUAN Y P. Transcriptome profiling of Huanglongbing ( HLB) tolerant and susceptible Citrus plants reveals the role of basal resistance in HLB tolerance. Frontiers in Plant Science, 2016, 7: Article 933. |
| [19] | 李湘龙, 柏斌, 吴俊, 邓启云, 周波 . 第二代测序技术用于水稻和稻瘟菌互作早期转录组的分析. 遗传, 2012,34(1):102-112. |
| LI X L, BAI B, WU J, DENG Q Y, ZHOU B . Transcriptome analysis of early interaction between rice and Magnaporthe oryzae using next-generation sequencing technology. Hereditas, 2012,34(1):102-112. (in Chinese) | |
| [20] |
KIM D, LANGMEAD B, SALZBERG S L . HISAT: A fast spliced aligner with low memory requirements. Nature Methods, 2015,12(4):357-360.
doi: 10.1038/nmeth.3317 pmid: 25751142 |
| [21] | MATSUKAWA M, SHIBATA Y, OHTSU M, MIZUTANI A, MORI H, WANG P, OJIKA M, KAWAKITA K, TAKEMOTO D . Nicotiana benthamiana calreticulin 3a is required for the ethylene-mediated production of phytoalexins and disease resistance against oomycete pathoge. Phytophthora infestans. Molecular Plant-Microbe Interactions, 2013,26(8):880-892. |
| [22] |
GUNDLACH H , MÜLLER M J, KUTCHAN T M, ZENK M H. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proceedings of the National Academy of Sciences of the United States of America, 1992,89(6):2389-2393.
doi: 10.1073/pnas.89.6.2389 pmid: 11607285 |
| [23] |
NOJIRI H, SUGIMORI M, YAMANE H, NISHIMURA Y, YAMADA A, SHIBUYA N, KODAMA O, MUROFUSHI N, OMORI T . Involvement of jasmonic acid in elicitor-induced phytoalexin production in suspension-cultured rice cells. Plant Physiology, 1996,110(2):387-392.
doi: 10.1104/pp.110.2.387 pmid: 12226190 |
| [24] |
VAN WEES S C M, CHANG H S, ZHU T, GLAZEBROOK J . Characterization of the early response of Arabidopsis t. Alternaria brassicicola infection using expression profiling. Plant Physiology, 2003,132(2):606-617.
doi: 10.1104/pp.103.022186 pmid: 12805591 |
| [25] | VAHABI K, REICHELT M, SCHOLZ S S, FURCH A C U, MATSUO M, JOHNSON J M, SHERAMETI I, GERSHENZON J , OELMULLER R. Alternaria brassicae induces systemic jasmonate responses in Arabidopsis which travel to neighboring plants via a Piriformsopora indica hyphal network and activate abscisic acid responses. Frontiers in Plant Science, 2018, 9: Article 626. |
| [26] |
DESMOND O J, EDGAR C I, MANNERS J M, MACLEAN D J, SCHENK P M, KAZAN K . Methyl jasmonate induced gene expression in wheat delays symptom development by the crown rot pathogen Fusarium pseudograminearum. Physiological and Molecular Plant Pathology, 2006,67:171-179.
doi: 10.1016/j.pmpp.2005.12.007 |
| [27] |
JAYARAJ J, MUTHUKRISHNAN S, LIANG G H, VELAZHAHAN R . Jasmonic acid and salicylic acid induce accumulation of β-1,3-glucanase and thaumatin-like proteins in wheat and enhance resistance against Stagonospora nodorum. Biologia Plantarum, 2004,48(3):425-430.
doi: 10.1023/B:BIOP.0000041097.03177.2d |
| [28] |
PANDEY S P, SOMSSICH I E . The role of WRKY transcription factors in plant immunity. Plant Physiology, 2009,150(4):1648-1655.
doi: 10.1104/pp.109.138990 pmid: 19420325 |
| [29] |
LI J, BRADER G, PALVA E T . The WRKY70 transcription factor: A node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. The Plant Cell, 2004,16(2):319-331.
doi: 10.1105/tpc.016980 pmid: 14742872 |
| [30] |
ABUQAMAR S, CHEN X, DHAWAN R, BLUHM B, SALMERON J, LAM S, DIETRICH R A, MENGISTE T . Expression profiling and mutant analysis reveals complex regulatory networks involved in Arabidopsis response t. Botrytis infection. The Plant Journal, 2006,48(1):28-44.
doi: 10.1111/j.1365-313X.2006.02849.x pmid: 16925600 |
| [31] |
ZHEN Z Y, QAMAR S A, CHEN Z X, MENGISTE T . Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. The Plant Journal, 2006,48(4):592-605.
doi: 10.1111/j.1365-313X.2006.02901.x pmid: 17059405 |
| [32] |
ANDREASSON E, JENKINS T, BRODERSEN P, THORGRIMSEN S, PETERSEN N H, ZHU S, QIU J L, MICHEELSEN P, ROCHER A, PETERSEN M, NEWMAN M A, NIELSEN H B, HIRT H, SOMSSICH I, MATTSSON O, MUNDY J . The map kinase substrate MKS1 is a regulator of plant defense responses. The EMBO Journal, 2005,24(14):2579-2589.
doi: 10.1038/sj.emboj.7600737 pmid: 15990873 |
| [33] |
QIU J L, FIIL B K, PETERSEN K, NIELSEN H B, BOTANGA C J, THORGRIMSEN S, PALMA K , SUAREZ-RODRIGUEZ M C, SANDBECH-CLAUSEN S, LICHOTA J, BRODERSEN P, GRASSER K D, MATTSSON O, GLAZEBROOK J, MUNDY J, PETERSEN M. Arabidopsis map kinase 4 regulates gene expression through transcription factor release in the nucleus. The EMBO Journal, 2008,27(16):2214-2221.
doi: 10.1038/emboj.2008.147 pmid: 18650934 |
| [34] |
LIU Y, XIN J, LIU L, SONG A, GUAN Z, FANG W, CHEN F . A temporal gene expression map of chrysanthemum leaves infected with Alternaria alternata reveals different stages of defense mechanisms. Horticulture Research, 2020,7:23.
doi: 10.1038/s41438-020-0245-0 pmid: 32140232 |
| [35] | FAN Q, SONG A, XIN J, CHEN S, JIANG J, WANG Y, LI X, CHEN F . Cmwrky15 facilitate. Alternaria tenuissima infection of chrysanthemum. PLoS ONE, 2015,10(11):e0143349. |
| [36] |
PRE M, ATALLAH M, CHAMPION A, DE VOS M, PIETERSE C M, MEMELINK J . The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense. Plant Physiology, 2008,147(3):1347-1357.
doi: 10.1104/pp.108.117523 pmid: 18467450 |
| [37] |
PIETERSE C M, LEON-REYES A , VAN DER ENT S, VAN WEES S C. Networking by small-molecule hormones in plant immunity. Nature Chemical Biology, 2009,5(5):308-316.
doi: 10.1038/nchembio.164 pmid: 19377457 |
| [38] |
GUTTERSON N, REUBER T L . Regulation of disease resistance pathways by AP2/ERF transcription factors. Current Opinion in Plant Biology, 2004,7(4):465-471.
doi: 10.1016/j.pbi.2004.04.007 |
| [39] | YU W Q, ZHAO R R, SHENG J P, SHEN L . SLERF2 is associated with methyl jasmonate-mediated defense response agains. Botrytis cinerea in tomato fruit. Journal of Agricultural and Food Chemistry, 2018,66(38):9923-9932. |
| [40] |
BERROCAL-LOBO M, MOLINA A . Ethylene response factor 1 mediates Arabidopsis resistance to the soilborne fungu. Fusarium oxysporum. Molecular Plant-Microbe Interactions, 2004,17(7):763-770.
doi: 10.1094/MPMI.2004.17.7.763 pmid: 15242170 |
| [41] |
BERROCAL-LOBO M, MOLINA A, SOLANO R . Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 i. Arabidopsis confers resistance to several necrotrophic fungi. The Plant Journal, 2002,29(1):23-32.
doi: 10.1046/j.1365-313x.2002.01191.x pmid: 12060224 |
| [42] |
LEON-REYES A, DU Y J, KOORNNEEF A, PROIETTI S, KORBES A P, MEMELINK J, PIETERSE C M, RITSEMA T . Ethylene signaling renders the jasmonate response of Arabidopsis insensitive to future suppression by salicylic acid. Molecular Plant-Microbe Interactions, 2010,23(2):187-197.
doi: 10.1094/MPMI-23-2-0187 pmid: 20064062 |
| [43] |
FERRER J L, AUSTIN M B, STEWART Jr . C, NOEL J P. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiology and Biochemistry, 2008,46(3):356-370.
doi: 10.1016/j.plaphy.2007.12.009 |
| [44] | ZHU L, NI W, LIU S, CAI B, XING H , WANG S. Transcriptomics analysis of apple leaves in response to Alternaria alternata apple pathotype infection. Frontiers in Plant Science, 2017, 8: Article 22. |
| [45] |
DORIA M S, GUEDES M S , DE ANDRADE SILVA E M, DE OLIVEIRA T M, PIROVANI C P, KUPPER K C, BASTIANEL M, MICHELI F. Comparative proteomics of two citrus varieties in response to infection by the fungus Alternaria alternata. International Journal of Biological Macromolecules, 2019,136:410-423.
doi: 10.1016/j.ijbiomac.2019.06.069 pmid: 31199975 |
| [46] |
SUN H H, WANG L, ZHANG B Q, MA J H, HETTENHAUSEN C, CAO G Y, SUN G L, WU J Q, WU J S . Scopoletin is a phytoalexin against Alternaria alternata in wild tobacco dependent on jasmonate signalling. Journal of Experimental Botany, 2014,65(15):4305-4315.
doi: 10.1093/jxb/eru203 |
| [47] |
THOLL D . Biosynthesis and biological functions of terpenoids in plants. Advances in Biochemical Engineering/Biotechnology, 2015,148:63-106.
doi: 10.1007/10_2014_295 pmid: 25583224 |
| [48] | SONG N, MA L, WANG W, SUN H, WANG L, BALDWIN I T, WU J S . An ERF2-like transcription factor regulates production of the defense sesquiterpene capsidiol upon Alternaria alternata infection. Journal of Experimental Botany, 2019,70(20):5895-5908. |
| [49] | CHEN X, CHEN H, YUAN J S, KOLLNER T G, CHEN Y, GUO Y, ZHUANG X, CHEN X, ZHANG Y J, FU J , NEBENFÜHR A, GUO Z, CHEN F. The rice terpene synthase gene OsTPS19 functions as an( S)-limonene synthase in planta, and its overexpression leads to enhanced resistance to the blast fungus Magnaporthe oryzae.Plant Biotechnology Journal 2018, 16(10):1778-1787. |
| [50] |
HAMMERBACHER A, COUTINHO T A, GERSHENZON J . Roles of plant volatiles in defense against microbial pathogens and microbial exploitation of volatiles. Plant, Cell and Environment, 2019,42(10):2827-2843.
doi: 10.1111/pce.13602 pmid: 31222757 |
| [51] | HUANG H, ULLAH F, ZHOU D X, YI M , ZHAO Y. Mechanisms of ROS regulation of plant development and stress responses. Frontiers in Plant Science, 2019, 10: Article 800. |
| [52] |
MARINO D, DUNAND C, PUPPO A, PAULY N . A burst of plant NADPH oxidases. Trends in Plant Science, 2012,17(1):9-15.
doi: 10.1016/j.tplants.2011.10.001 |
| [53] |
TORRES M A . ROS in biotic interactions. Physiologia Plantarum, 2010,138(4):414-429.
doi: 10.1111/j.1399-3054.2009.01326.x pmid: 20002601 |
| [54] | KUŹNIAK E . The ascorbate-gluathione cycle and related redox signals in plant pathogen interactions//ANJUM N A. Ascorbate- Glutathione Pathway and Stress Tolerance in Plants. Springer Science+Business Media B. V, 2010: 115-136. |
| 责任编辑: 岳梅 |
| [1] | 由玉婉,张雨,孙嘉毅,张蔚. ‘月月粉’月季NAC家族全基因组鉴定及皮刺发育相关成员的筛选[J]. 中国农业科学, 2022, 55(24): 4895-4911. |
| [2] | 尤佳玲,李有梅,孙孟豪,谢兆森. ‘黑比诺’葡萄不同叶龄叶片叶绿体内淀粉积累及其相关基因表达差异分析[J]. 中国农业科学, 2022, 55(21): 4265-4278. |
| [3] | 孙保娟,汪瑞,孙光闻,王益奎,李涛,宫超,衡周,游倩,李植良. 转录组及代谢组联合解析茄子果色上位遗传效应[J]. 中国农业科学, 2022, 55(20): 3997-4010. |
| [4] | 刘鑫,张亚红,袁苗,党仕卓,周娟. ‘红地球’葡萄花芽分化过程中的转录组分析[J]. 中国农业科学, 2022, 55(20): 4020-4035. |
| [5] | 徐献斌,耿晓月,李慧,孙丽娟,郑焕,陶建敏. 基于转录组分析ABA促进葡萄花青苷积累相关基因[J]. 中国农业科学, 2022, 55(1): 134-151. |
| [6] | 郭永春, 王鹏杰, 金珊, 侯炳豪, 王淑燕, 赵峰, 叶乃兴. 基于WGCNA鉴定茶树响应草甘膦相关的基因共表达模块[J]. 中国农业科学, 2022, 55(1): 152-166. |
| [7] | 陈华枝,范元婵,蒋海宾,王杰,范小雪,祝智威,隆琦,蔡宗兵,郑燕珍,付中民,徐国钧,陈大福,郭睿. 基于纳米孔全长转录组数据完善东方蜜蜂微孢子虫的基因组注释[J]. 中国农业科学, 2021, 54(6): 1288-1300. |
| [8] | 杜宇,祝智威,王杰,王秀娜,蒋海宾,范元婵,范小雪,陈华枝,隆琦,蔡宗兵,熊翠玲,郑燕珍,付中民,陈大福,郭睿. 利用第三代纳米孔长读段测序技术构建和注释蜜蜂球囊菌的全长转录组[J]. 中国农业科学, 2021, 54(4): 864-876. |
| [9] | 赵卫松,郭庆港,董丽红,王培培,苏振贺,张晓云,鹿秀云,李社增,马平. 枯草芽孢杆菌NCD-2对棉花根系分泌物L-脯氨酸响应的转录-蛋白质组学联合分析[J]. 中国农业科学, 2021, 54(21): 4585-4600. |
| [10] | 刘恋,唐志鹏,李菲菲,熊江,吕壁纹,马小川,唐超兰,李泽航,周铁,盛玲,卢晓鹏. ‘融安金柑’‘滑皮金柑’及‘脆蜜金柑’贮藏期品质、贮藏特性及果皮转录组分析[J]. 中国农业科学, 2021, 54(20): 4421-4433. |
| [11] | 刘锴,何闪闪,张彩霞,张利义,卞书迅,袁高鹏,李武兴,康立群,丛佩华,韩晓蕾. 苹果叶片不定芽再生过程的差异表达基因鉴定与分析[J]. 中国农业科学, 2021, 54(16): 3488-3501. |
| [12] | 林兵,陈艺荃,钟淮钦,叶秀仙,樊荣辉. 荷兰鸢尾‘玉妃’花色变异关键结构基因分析[J]. 中国农业科学, 2021, 54(12): 2644-2652. |
| [13] | 秦秋红,何旭江,江武军,王子龙,曾志将. 东方蜜蜂幼虫封盖信息素含量及生物合成通路[J]. 中国农业科学, 2021, 54(11): 2464-2475. |
| [14] | 龙琴,杜美霞,龙俊宏,何永睿,邹修平,陈善春. 转录因子CsWRKY61对柑橘溃疡病抗性的影响[J]. 中国农业科学, 2020, 53(8): 1556-1571. |
| [15] | 滕彩玲,钟晰,吴昊娣,胡燕,周常勇,王雪峰. 马蜂柑响应黄龙病菌不同侵染时期的生物学和转录组学分析[J]. 中国农业科学, 2020, 53(7): 1368-1380. |
|
||