













中国农业科学 ›› 2020, Vol. 53 ›› Issue (8): 1556-1571.doi: 10.3864/j.issn.0578-1752.2020.08.006
收稿日期:2019-09-26
接受日期:2019-11-13
出版日期:2020-04-16
发布日期:2020-04-29
通讯作者:
邹修平,陈善春
作者简介:龙琴,E-mail: longlong860923@126.com。
基金资助:
LONG Qin,DU MeiXia,LONG JunHong,HE YongRui,ZOU XiuPing( ),CHEN ShanChun(
),CHEN ShanChun( )
)
Received:2019-09-26
Accepted:2019-11-13
Online:2020-04-16
Published:2020-04-29
Contact:
XiuPing ZOU,ShanChun CHEN
摘要:
【背景】柑橘溃疡病(citrus bacterial canker,CBC)是世界柑橘产业上危害最严重的病害之一,由柑橘黄单胞杆菌柑橘亚种(Xanthomonas citri subsp. citri,Xcc)引起,在国内外被列为检疫对象。由于柑橘分子病理研究相对滞后,导致可供利用的抗性基因资源相对匮乏。WRKY转录因子参与植物抵御生物和非生物胁迫反应,前期研究发现柑橘WRKY转录因子可能在调控寄主抗病反应中起着重要作用。【目的】通过对超量表达CsWRKY50、CsWRKY61和CsWRKY72转基因晚锦橙(Citrus sinensis)的溃疡病抗性进行评价,明确这些基因在柑橘响应溃疡病菌侵染中的生物学功能和抗病育种价值。进一步利用RNA-Seq解析CsWRKY61调控的信号通路。【方法】利用农杆菌介导法进行柑橘遗传转化,获得超量表达CsWRKY50、CsWRKY61和CsWRKY72的晚锦橙;利用实时荧光定量PCR(qRT-PCR)分析转基因植株中目的基因的表达水平以及拷贝数;以非转基因植株为对照,采用离体针刺接种评价转基因植株对溃疡病的抗性;通过比较超量表达CsWRKY61和野生型植株的转录组测序结果,探究CsWRKY61提高柑橘溃疡病抗性的内在机制。【结果】分别构建了CAMV 35S启动子控制CsWRKY50、CsWRKY61和CsWRKY72表达的植物表达载体,通过GUS组织化学染色和PCR鉴定分别获得了6、8和6株转基因晚锦橙。转基因植株中目的基因的表达量有不同程度的提高,大部分转基因植株中外源基因的拷贝数为1,只有超量表达CsWRKY61的转基因植株溃疡病抗性显著增强,其病斑面积明显小于野生型植株,而超量表达CsWRKY50和CsWRKY72的转基因植株抗病性与野生型相比无明显差异。转录组分析结果显示,超量表达CsWRKY61的转基因植株中生物胁迫相关途径(包括病原入侵的感知、活性氧爆发、转录因子、防御基因、激素、细胞壁和次生代谢等)和信号转导相关途径(主要是激酶受体)均被显著激活。【结论】超量表达CsWRKY61能够激活与生物胁迫和信号转导相关的途径,增强柑橘对溃疡病的抗性;CsWRKY61在柑橘抗病育种中存在潜在的应用价值。
龙琴,杜美霞,龙俊宏,何永睿,邹修平,陈善春. 转录因子CsWRKY61对柑橘溃疡病抗性的影响[J]. 中国农业科学, 2020, 53(8): 1556-1571.
LONG Qin,DU MeiXia,LONG JunHong,HE YongRui,ZOU XiuPing,CHEN ShanChun. Effect of Transcription Factor CsWRKY61 on Citrus Bacterial Canker Resistance[J]. Scientia Agricultura Sinica, 2020, 53(8): 1556-1571.
表1
基因克隆和转基因植株鉴定所用引物"
| 引物名称 Primer name | 引物序列 Primer sequence (5′-3′) |
|---|---|
| WRKY50-f | ATC GGATCC ATGTCTAATATAAATCTTAT |
| WRKY50-r | CTG GTCGAC CTAAGGATTGCTTTGGTGAG |
| WRKY61-f | ACC AGATCT ATGGAGGAGAAAAGAGCTAT |
| WRKY61-r | TTA GTCGAC TCAGTCAGTGTGCTCTCTAT |
| WRKY72-f | TAG GGATCC ATGGAGGTTTTATTGAAAATG |
| WRKY72-r | CAT GTCGAC TCAACTGCTTTGATCCTTGT |
| NPTII-f | GGATTGCACGCAGGTTCTCCG |
| NPTII-r | TCAGAAGAACTCGTCAAGAAGGCG |
| s-f | TCTTCGTCAACATGGTGGAGCACGA |
| 50-r | ACTCATCAAAGGTCAAGTACTCAG |
| 61-r | TCTGGCAGTTTCAAGCTGATCATC |
| 72-r | ACAAGTTCAGACTCCATGATTTGAC |
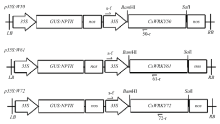
图1
植物表达载体T-DNA结构示意图 35S:花椰菜花叶病毒CaMV 35S启动子Cauliflower mosaic virus 35S promoter (CaMV 35S);GUS:NPTII:β-葡萄糖醛酸酶GUS报告基因与卡那霉素NPTII抗性基因的融合基因The fusion gene of β-glucuronidase and neomycin phosphotransferase genes;nos:胭脂碱合酶基因的转录终止序列The terminator of the nopaline synthase gene。单箭头表示转基因植株鉴定用引物 Arrow indicates the primer used to determine transgenic plant"
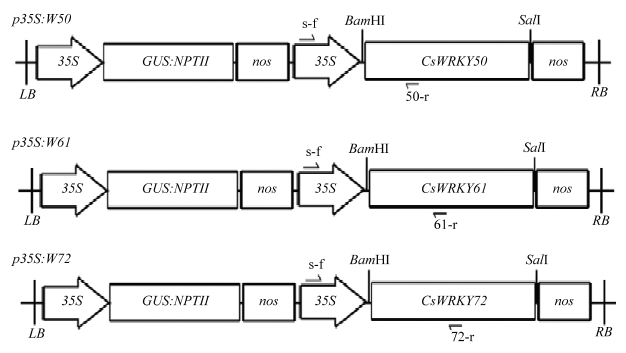
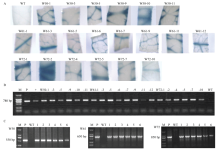
图2
转基因植株鉴定 A:转基因植株的GUS组织化学染色图GUS histochemical staining of transgenic plants;B:转基因植株NPTII扩增结果Amplification results of NPTII in transgenic plants;C:部分转基因植株目的基因的PCR扩增PCR amplification of target genes in some transgenic plants。M:DNA marker;P:质粒模板Plasmid template;+:已鉴定的阳性植株对照Identified positive plant control"
表3
转基因植株外源基因拷贝数实时荧光定量PCR分析"
| 株系 Line | 2-ΔΔCt值2-ΔΔCt value | 预测拷贝数 Estimated copy number | |
|---|---|---|---|
| GUS | NPTII | ||
| W50-1 | 1.32 | 1.39 | 1 |
| W50-3 | 1.50 | 1.26 | 1 |
| W50-5 | 1.90 | 1.64 | 2 |
| W50-9 | 3.56 | 3.72 | 4 |
| W50-10 | 2.84 | 2.76 | 3 |
| W50-11 | 2.84 | 2.51 | 3 |
| W61-1 | 1.07 | 1.47 | 1 |
| W61-3 | 1.14 | 1.55 | 1 |
| W61-5 | 1.07 | 0.98 | 1 |
| W61-6 | 1.33 | 1.63 | 1 |
| W61-7 | 1.13 | 1.14 | 1 |
| W61-9 | 0.82 | 1.05 | 1 |
| W61-11 | 0.97 | 0.82 | 1 |
| W61-12 | 1.22 | 1.32 | 1 |
| W72-1 | 2.57 | 2.41 | 2 |
| W72-2 | 1.62 | 1.90 | 2 |
| W72-4 | 2.68 | 2.96 | 3 |
| W72-5 | 1.28 | 1.30 | 1 |
| W72-7 | 1.32 | 1.49 | 1 |
| W72-10 | 1.04 | 1.12 | 1 |
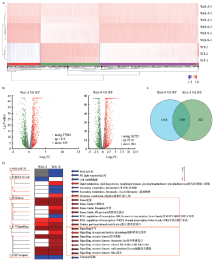
图5
转录组结果总概括 A:差异基因表达分析聚类热图。红色表示上调,蓝色表示下调Cluster heat map for differential gene expression analysis. Red and blue indicate up-regulated and down-regulated, respectively。B:差异基因表达分析火山图。红色表示上调,绿色表示下调Volcano map of differential gene expression analysis. Red and green indicate up-regulated and down-regulated, respectively。C:差异基因表达分析维恩图。中间重叠部分代表共有的差异基因Venn diagram of differential gene expression analysis. The middle overlap represents the shared genes。D:差异表达基因的MapMan可视化分析。红色表示上调,蓝色表示下调MapMan visual analysis of differential genes. Red and blue indicate up-regulated and down-regulated, respectively"

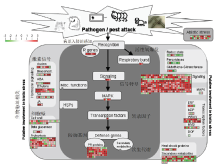
图6
生物胁迫相关的差异基因情况 该图为MapMan注释的转基因株系W61-9中与生物胁迫相关的差异基因情况。每个方块表示一个基因,红色表示上调表达,绿色表示下调表达The figure shows the differential genes involved in biotic stress of W61-9 transgenic line by MapMan. Each square represents one gene. Red and green indicate up-regulated expression and down-regulated expression, respectively"
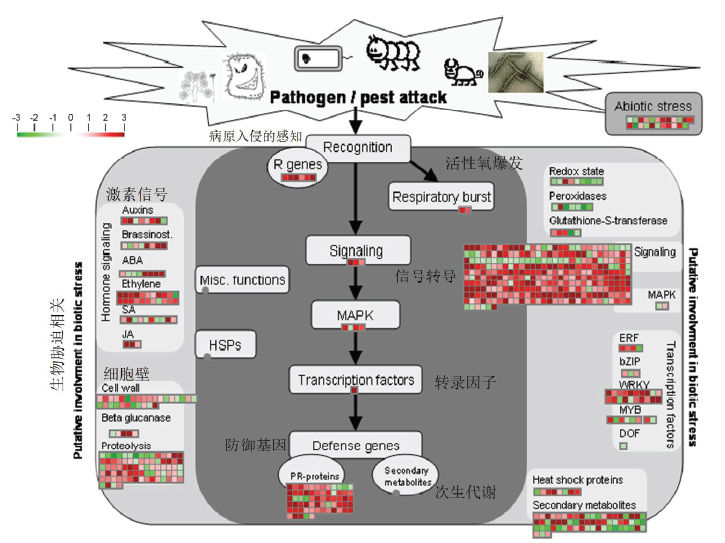
表4
转基因植株中生物胁迫相关的差异基因情况"
| 通路Pathway | 基因编号Gene ID | 描述Description | Log2 fold change |
|---|---|---|---|
| 感知Recognition | cs7g19210 | 抗病蛋白家族Disease resistance protein (TIR-NBS-LRR class) family | 2.464 |
| orange1.1t04440 | 抗病蛋白家族Disease resistance protein (TIR-NBS-LRR class) family | 2.839 | |
| orange1.1t05036 | Toll-Interleukin-Resistance (TIR) domain family protein | 3.153 | |
| cs5g18230 | Toll-Interleukin-Resistance (TIR) domain family protein | 3.639 | |
| 活性氧爆发Respiratory burst | cs4g06920 | NADPH/respiratory burst oxidase protein D (RbohD) | 1.962 |
| cs8g12000 | 核黄素合成酶样超家族蛋白Riboflavin synthase-like superfamily protein | 2.631 | |
| 信号转导 Signaling transduction | orange1.1t03802 | alpha/beta-Hydrolases superfamily protein | 3.683 |
| cs5g04790 | alpha/beta-Hydrolases superfamily protein | 2.423 | |
| cs6g07420 | PAR1 protein | 3.644 | |
| cs7g07000 | 假定的配体门控离子通道亚家族Putative ligand-gated ion channel subunit family | 3.786 | |
| cs1g16140 | 富亮氨酸重复I Leucine rich repeat I | 4.144 | |
| orange1.1t03347 | 富亮氨酸重复II Leucine rich repeat II | 3.177 | |
| orange1.1t04450 | 富亮氨酸重复VII Leucine rich repeat VII | 3.279 | |
| cs2g29910 | 富亮氨酸重复XII Leucine rich repeat XII | 4.256 | |
| orange1.1t04450 | 富亮氨酸重复XI Leucine rich repeat XI | 3.279 | |
| cs2g29890 | 富亮氨酸重复XIII Leucine rich repeat XIII | 2.459 | |
| cs2g13280 | 奇异果甜蛋白受体激酶Thaumatin like receptor kinases | 3.153 | |
| cs2g13360 | 奇异果甜蛋白受体激酶Thaumatin like receptor kinases | 3.199 | |
| cs1g11930 | DUF 26受体激酶DUF 26 receptor kinases | 3.791 | |
| cs1g11960 | DUF 26受体激酶DUF 26 receptor kinases | 3.052 | |
| cs8g12370 | 豆科凝集素Legume-lectin | 5.351 | |
| cs2g13360 | Wheat LRK10 like | 3.199 | |
| cs1g11930 | S位点糖蛋白S-locus glycoprotein like | 3.791 | |
| cs1g11960 | S位点糖蛋白S-locus glycoprotein like | 3.052 | |
| cs8g01090 | 豆科凝集素Legume-lectin | 2.194 | |
| cs8g14010 | 胞壁相关激酶Wall associated kinase | 3.464 | |
| cs9g12300 | 胞壁相关激酶Wall associated kinase | 3.013 | |
| cs9g14490 | 胞壁相关激酶Wall associated kinase | 3.918 | |
| cs9g08050 | 赖氨酸基序Lysine motif | 1.079 | |
| cs2g02680 | 赖氨酸基序Lysine motif | 2.853 | |
| cs7g31060 | 褶皱样Crinkly like | 2.283 | |
| cs5g17510 | 褶皱样Crinkly like | 1.589 | |
| cs9g12040 | Ralf-like 32 (RALFL32) | 5.157 | |
| cs9g12160 | Ralf-like 32 (RALFL32) | 3.365 | |
| cs7g27120 | 钙调蛋白结合蛋白Calmodulin binding protein-like | 3.590 | |
| cs9g02980 | 钙调蛋白结合蛋白Calmodulin binding protein-like | 3.336 | |
| cs2g21150 | 蛋白激酶激酶Mitogen-activated protein kinase kinase | 2.077 | |
| cs3g27320 | 受体样胞浆激酶VII Receptor like cytoplasmatic kinase VII | 3.278 | |
| cs9g15620 | 受体样胞浆激酶VII Receptor like cytoplasmatic kinase VII | 4.530 | |
| cs6g20470 | AAA ATPase 1 | 5.065 | |
| cs5g18300 | 天冬氨酸蛋白酶Aspartate protease | 4.689 | |
| orange1.1t03718 | 半胱氨酸蛋白酶Cysteine protease | 3.775 | |
| cs2g19970 | F-box和相关的相互作用域包含蛋白质 F-box and associated interaction domains-containing protein | 4.369 | |
| cs2g31260 | F-box和相关的相互作用域包含蛋白质 F-box and associated interaction domains-containing protein | 7.072 | |
| 转录因子 Transcription factor | orange1.1t02158 | 富含亮氨酸的重复单位Leucine-rich repeat | 1.487 |
| cs5g24240 | 抗病蛋白Disease resistance protein (TIR-NBS-LRR class) | 3.080 | |
| cs5g04160 | WRKY61 DNA结合蛋白WRKY DNA-binding protein 61 | 10.442 | |
| cs1g03870 | WRKY51 DNA结合蛋白WRKY DNA-binding protein 51 | 7.885 | |
| cs7g06330 | WRKY40 DNA结合蛋白WRKY DNA-binding protein 40 | 3.707 | |
| cs4g07780 | R2R3 MYB 转录因子基因家族R2R3 MYB transcription factor gene family | 6.169 | |
| cs8g12680 | 转录因子RLTR1 Transcription factor RLTR1 | 2.614 | |
| 防御基因 Defense gene | orange1.1t04673 | 抗病蛋白Disease resistance protein | 3.487 |
| orange1.1t04706 | 抗病蛋白Disease resistance protein (TIR-NBS-LRR class) | 2.892 | |
| orange1.1t04750 | Toll-Interleukin receptor | 3.454 | |
| orange1.1t05036 | 抗病蛋白Disease resistance protein (TIR-NBS-LRR class) | 3.153 | |
| orange1.1t05250 | Toll-Interleukin receptor | 3.423 | |
| cs2g10790 | NPR1-like protein 3 | 1.251 |
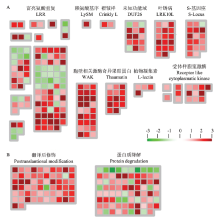
图7
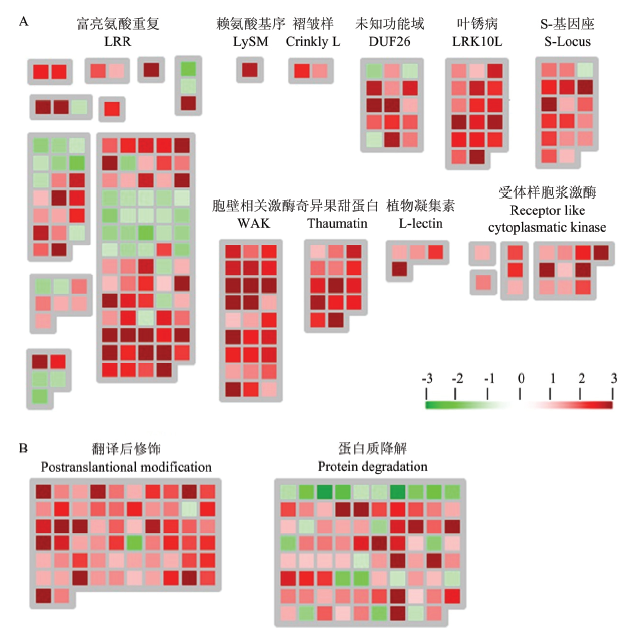
受体样激酶和蛋白质代谢相关基因的表达情况 A:W61-9中受体样激酶相关基因的表达情况Differential expression of receptor-like kinase-related genes of W61-9 line;B:W61-9中蛋白质代谢相关基因的表达情况Differential expression of genes related to protein metabolism of W61-9 line该结果通过MapMan分析。每个方块表示一个基因,红色表示上调表达,绿色表示下调表达The results were analyzed by MapMan. Each square represents one gene. Red and green indicate up-regulated expression and down-regulated expression, respectively"
| [1] | BRUNINGS A M, GABRIEL D W . Xanthomonas citri: breaking the surface. Molecular Plant Pathology, 2003,4(3):141-157. |
| [2] | SCHUBERT T S, RIZVI S A, SUN X, GOTTWALD T R, GRAHAM J H, DIXON W N . Meeting the challenge of eradicating citrus canker in Florida—again. Plant Disease, 2001,85(4):340-356. |
| [3] | BEHLAU F, BELASQUE J, BERGAMIN FILHO A, GRAHAM J H, LEITE R P, GOTTWALD T R . Copper sprays and windbreaks for control of citrus canker on young orange trees in southern Brazil. Crop Protection, 2008,27(3/5):807-813. |
| [4] | DAS A K . Citrus canker-A review. Journal of Applied Horticulture, 2003,5(1):52-60. |
| [5] | BAKSHI M, OELMÜLLER R . WRKY transcription factors: Jack of many trades in plants. Plant Signaling and Behavior, 2014,9(2):e27700. |
| [6] | EULGEM T, RUSHTON P J, ROBATZEK S, SOMSSICH I E . The WRKY superfamily of plant transcription factors. Trends in Plant Science, 2000,5(5):199-206. |
| [7] | EULGEM T, SOMSSICH I E . Networks of WRKY transcription factors in defense signaling. Current Opinion in Plant Biology, 2007,10(4):366-371. |
| [8] | ROSS C A, LIU Y, SHEN Q J . The WRKY gene family in rice (Oryza sativa). Journal of Intergative Plant Biology, 2007,49(6):827-842. |
| [9] | JIANG Y, DUAN Y, YIN J, YE S, ZHU J, ZHANG F, LU W, FAN D, LUO K . Genome-wide identification and characterization of the Populus WRKY transcription factor family and analysis of their expression in response to biotic and abiotic stresses. Journal of Experimental Botany, 2014,65(22):6629-6644. |
| [10] | 刁卫平, 王述彬, 刘金兵, 潘宝贵, 郭广君, 戈伟 . 辣椒全基因组WRKY转录因子的分析. 园艺学报, 2015,42(11):2183-2196. |
| DIAO W P, WANG S B, LIU J B, PAN B G, GUO G J, GE W . Gemone-wide analysis of the WRKY transcription factor family in pepper. Acta Horticulturae Sinica, 2015,42(11):2183-2196. (in Chinese) | |
| [11] | 许瑞瑞, 张世忠, 曹慧, 束怀瑞 . 苹果WRKY转录因子家族基因生物信息学分析. 园艺学报, 2012,39(10):2049-2060. |
| XU R R, ZHANG S Z, CAO H, SHU H R . Bioinformatics analysis of WRKY transcription factor genes family in apple. Acta Horticulturae Sinica, 2012,39(10):2049-2060. (in Chinese) | |
| [12] | PHUKAN U J, JEENA G S, SHUKLA R K . WRKY transcription factors: Molecular regulation and stress responses in plants. Frontiers in Plant Science, 2016, 7: Article 760. |
| [13] | DANG F F, WANG Y N, YU L, EULGEM T, LAI Y, LIU Z Q, WANG X, QIU A L, ZHANG T X, LIN J, CHEN Y S, GUAN D Y, CAI H Y, MOU S L, HE S L . CaWRKY40, a WRKY protein of pepper, plays an important role in the regulation of tolerance to heat stress and resistance to Ralstonia solanacearum infection. Plant, Cell and Environment, 2013,36(4):757-774. |
| [14] | MIAO Y, JIANG J, REN Y, ZHAO Z . The single-stranded DNA-binding protein WHIRLY1 represses WRKY53 expression and delays leaf senescence in a developmental stage-dependent manner in Arabidopsis. Plant Physiology, 2013,163(2):746-756. |
| [15] | PANDEY S P, ROCCARO M, SCHÖN M, LOGEMANN E, SOMSSICH I E . Transcriptional reprogramming regulated by WRKY18 and WRKY40 facilitates powdery mildew infection of Arabidopsis. The Plant Journal for Cell and Molecular Biology, 2010,64(6):912-923. |
| [16] | QIU Y P, YU D Q . Over-expression of the stress-induced OsWRKY45 enhances disease resistance and drought tolerance in Arabidopsis. Environmental and Experimental Botany, 2009,65(1):35-47. |
| [17] | KLOTH K J, WIEGERS G L, BUSSCHER-LANGE J, VAN HAARST J C, KRUIJER W, BOUWMEESTER H J, DICKE M, JONGSMA M A . AtWRKY22 promotes susceptibility to aphids and modulates salicylic acid and jasmonic acid signalling. Journal of Experimental Botany, 2016,67(11):3383-3396. |
| [18] | ABBRUSCATO P, NEPUSZ T, MIZZI L, CORVO M D, MORANDINI P, FUMASONI I, MICHEL C, PACCANARO A, GUIDERDONI E, SCHAFFRATH U, MOREL J B, PIFFANELLI P, FAIVRE-RAMPANT O . OsWRKY22, a monocot WRKY gene, plays a role in the resistance response to blast. Molecular Plant Pathology, 2012,13(8):828-841. |
| [19] | KIM K C, LAI Z, FAN B, CHEN Z . Arabidopsis WRKY38 and WRKY62 transcription factors interact with histone deacetylase 19 in basal defense. The Plant Cell, 2008,20(9):2357-2371. |
| [20] | LAI Z, VINOD K M, ZHENG Z, FAN B, CHEN Z . Roles of Arabidopsis WRKY3 and WRKY4 transcription factors in plant responses to pathogens. BMC Plant Biology, 2008,8:68. |
| [21] | LIU J, CHEN X, LIANG X, ZHOU X, YANG F, LIU J, HE S Y, GUO Z . Alternative splicing of rice WRKY62 and WRKY76 transcription factor genes in pathogen defense. Plant Physiology, 2016,171(2):1427-1442. |
| [22] | LIU S, KRACHER B, ZIEGLER J, BIRKENBIHL R P, SOMSSICH I E . Negative regulation of ABA signaling by WRKY33 is critical for Arabidopsis immunity towards Botrytis cinerea 2100. eLife, 2015,4:e07295. |
| [23] | LIU X, BAI X, WANG X, CHU C . OsWRKY71, a rice transcription factor, is involved in rice defense response. Journal of Plant Physiology, 2007,164(8):969-979. |
| [24] | WU K L, GUO Z J, WANG H H, LI J . The WRKY family of transcription factors in rice and Arabidopsis and their origins. DNA Research, 2005,12:9-26. |
| [25] | PANDEY S P, SOMSSICH I E . The role of WRKY transcription factors in plant immunity. Plant Physiology, 2009,150(4):1648-1655. |
| [26] | ÜLKER B, MUKHTAR M S, SOMSSICH I E . The WRKY70 transcription factor of Arabidopsis influences both the plant senescence and defense signaling pathways. Planta, 2007,226(1):125-137. |
| [27] | XU X, CHEN C, FAN B, CHEN Z . Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. The Plant Cell, 2006,18(5):1310-1326. |
| [28] | ZHOU X, JIANG Y, YU D . WRKY22 transcription factor mediates dark-induced leaf senescence in Arabidopsis. Molecules and Cells, 2011,31(4):303-313. |
| [29] | SHI Q, FEBRES V J, JONES J B, MOORE G A . Responsiveness of different citrus genotypes to the Xanthomonas citri ssp. citri-derived pathogen-associated molecular pattern (PAMP) flg22 correlates with resistance to citrus canker. Molecular Plant Pathology, 2015,16(5):507-520. |
| [30] | VIVES-PERIS V, MARMANEU D, GÓMEZ-CADENAS A, PÉREZ-CLEMENTE R M . Characterization of Citrus WRKY transcription factors and their responses to phytohormones and abiotic stresses. Biologia Plantarum, 2018,62(1):33-44. |
| [31] | AYADI M, HANANA M, KHARRAT N, MERCHAOUI H, MARZOUG R B, LAUVERGEAT V, REBAÏ A, MZID R . The WRKY transcription factor family in citrus: Valuable and useful candidate genes for citrus breeding. Applied Biochemistry and Biotechnology, 2016,180(3):516-543. |
| [32] | ŞAHIN-ÇEVIK M, MOORE G A . Identification of a drought- and cold-stress inducible wrky gene in the cold-hardy citrus relative Poncirus trifoliata. New Zealand Journal of Crop and Horticultural Science, 2013,41(2):57-68. |
| [33] | ZOU X, LI D, LUO X, LUO K, PEI Y . An improved procedure for Agrobacterium-mediated transformation of trifoliate orange (Poncirus trifoliata L. Raf.) via indirect organogenesis. In Vitro Cellular and Developmental Biology Plant, 2008,44(3):169-177. |
| [34] | 周鹏飞 . 柑橘溃疡病相关WRKY转录因子和PR基因的筛选与功能分析[D]. 重庆: 西南大学, 2017. |
| ZHOU P F . Screening and functional analysis of WRKY transcription factor and pathogenesis-related protein genes associated with citrus canker[D]. Chongqing: Southwest University, 2017. (in Chinese) | |
| [35] | 王军政 . Cecropin B分泌型融合基因的构建及其转化柑桔的研究[D]. 重庆: 西南大学, 2012. |
| WANG J Z . Construction and citrus transformation of novel cecropin B gene for improving extracellualr secretion of antibacterial peptide[D]. Chongqing: Southwest University, 2012. (in Chinese) | |
| [36] | JEFFERSON R A, KAVANAGH T A, BEVAN M W . Gus fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. The EMBO Journal, 1987,6(13):3901-3907. |
| [37] | 许兰珍, 何永睿, 雷天刚, 彭爱红, 姚利晓, 姜国金, 李强, 邹修平, 陈善春 . 转基因柑橘外源基因拷贝数的实时荧光定量PCR检测. 园艺学报, 2016,43(6):1186-1194. |
| XU L Z, HE Y R, LEI T G, PENG A H, YAO L X, JIANG G J, LI Q, ZOU X P, CHEN S C . Identification of the copy number of exogenous gene in transgenic citurs by quantitative real-time PCR. Acta Horticulturae Sinica, 2016,43(6):1186-1194. (in Chinese) | |
| [38] | WU Z, BURNS J K . Isolation and characterization of a cDNA encoding a lipid transfer protein expressed in ‘Valencia’ orange during abscission. Journal of Experimental Botany, 2003,54(385):1183-1191. |
| [39] | 李云锋, 李祥 . 柑桔溃疡病菌存活期的研究. 植物检疫, 2002,16(2):69-72, 77. |
| LI Y F, LI X . Survival period of Xanthomonas axonopodis pv. citri. Plant Quarantine, 2002,16(2):69-72, 77. (in Chinese) | |
| [40] | 李云锋, 李祥 . 柑桔溃疡病菌离体叶接种检验法的研究. 华中农业大学学报, 2000,19(5):421-423. |
| LI Y F, LI X . Detection method of inoculation on citurs leaves in vitro with Xanthomonas axonopodis pv. citri. Journal of Huazhong Agricultural University, 2000,19(5):421-423. (in Chinese) | |
| [41] | THIMM O, BLASING O, GIBON Y, NAGEL A, MEYER S, KRUGER P, SELBIG J, MULLER L A, RHEE S Y, STITT M . MAPMAN: A user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. The Plant Journal, 2004,37(6):914-939. |
| [42] | WANG H, HAO J, CHEN X, HAO Z, WANG X, LOU Y, PENG Y, GUO Z . Overexpression of rice WRKY89 enhances ultraviolet B tolerance and disease resistance in rice plants. Plant Molecular Biology, 2007,65(6):799-815. |
| [43] | ZHENG Z, QAMAR S A, CHEN Z, MENGISTE T . Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. The Plant Journal, 2006,48(4):592-605. |
| [44] | WANG X, GUO R, TU M, WANG D, GUO C, WAN R, LI Z, WANG X . Ectopic expression of the wild grape WRKY transcription factor VqWRKY52 in Arabidopsis thaliana enhances resistance to the biotrophic pathogen powdery mildew but not to the necrotrophic pathogen Botrytis cinerea. Frontiers in Plant Science, 2017, 8: Article 97. |
| [45] | 朱镜如 . 毛果杨PtWRKY89转录因子基因的克隆与功能分析[D]. 重庆: 西南大学, 2013. |
| ZHU J R . Isolation and functional analysis of PtWRKY89 in Populus trichocarpa. Chongqing: Southwest University, 2013. (in Chinese) | |
| [46] | HEATH M C . Hyper sensitive response-related death. Plant Molecular Biology, 2000,44(3):321-334. |
| [47] | GREENBERG J T, YAO N . The role and regulation of programmed cell death in plant-pathogen interactions. Cellular Microbiology, 2004,6(3):201-211. |
| [48] | ROBERT-SEILANIANTZ A, GRANT M, JONES J D G . Hormone crosstalk in plant disease and defense: More than just jasmonate- salicylate antagonism. Annual Review of Phytopathology, 2011,49:317-343. |
| [49] | HÜCKELHOVEN R . Cell wall-associated mechanisms of disease resistance and susceptibility. Annual Review of Phytopathology, 2007,45:101-127. |
| [50] | VAIRAPPAN C S, ANANGDAN S P, KAI L T, MATSUNAGA S . Role of secondary metabolites as defense chemicals against ice-ice disease bacteria in biofouler at carrageenophyte farms. Journal of Applied Phycology, 2010,22(3):305-311. |
| [51] | 郭艳玲, 张鹏英, 郭默然, 陈靠山 . 次生代谢产物与植物抗病防御反应. 植物生理学报, 2012,48(5):429-434. |
| GUO Y L, ZHANG P Y, GUO M R, CHEN K S . Secondary metabolites and plant defence against pathogenic disease. Plant Physiology Journal, 2012,48(5):429-434. (in Chinese) | |
| [52] | YANG K Y, LIU Y, ZHANG S . Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proceedings of the National Academy of Sciences of the United States of America, 2001,98(2):741-746. |
| [53] | GAO R, LIU P, YONG Y, WONG S M . Genome-wide transcriptomic analysis reveals correlation between higher WRKY61 expression and reduced symptom severity in Turnip crinkle virus infected Arabidopsis thaliana. Scientific Reports, 2016,6:24604. |
| [54] | YAN L, QI X, YOUNG N D, OLSEN K M, CAICEDO A L, JIA Y . Characterization of resistance genes to rice blast fungus Magnaporthe oryzae in a “Green Revolution” rice variety. Molecular Breeding, 2015,35:52. |
| [55] | 范晓江, 郭小华, 牛芳芳, 杨博, 江元清 . 拟南芥WRKY61转录因子的转录活性与互作蛋白分析. 西北植物学报, 2018,38(1):1-8. |
| FAN X J, GUO X H, NIU F F, YANG B, JIANG Y Q . Exploring the transcriptional activity and interacting proteins of WRKY61 transcriptional factor in Arabidopsis thaliana. Acta Botanica Boreali-Occidentalia Sinica, 2018,38(1):1-8. (in Chinese) | |
| [56] | GAO Q M, VENUGOPAL S, NAVARRE D, KACHROO A . Low oleic acid-derived repression of jasmonic acid-inducible defense responses requires the WRKY50 and WRKY51 proteins. Plant Physiology, 2011,155(1):464-476. |
| [57] | BHATTARAI K K, ATAMIAN H S, KALOSHIAN I, EULGEM T . WRKY72-type transcription factors contribute to basal immunity in tomato and Arabidopsis as well as gene-for-gene resistance mediated by the tomato R gene Mi-1. The Plant Journal, 2010,63(2):229-240. |
| [1] | 郭泽西,孙大运,曲俊杰,潘凤英,刘露露,尹玲. 查尔酮合成酶基因在葡萄抗灰霉病和霜霉病中的作用[J]. 中国农业科学, 2022, 55(6): 1139-1148. |
| [2] | 胡朝月, 王凤涛, 郎晓威, 冯晶, 李俊凯, 蔺瑞明, 姚小波. 小麦抗条锈病基因对中国条锈菌主要流行小种的抗性分析[J]. 中国农业科学, 2022, 55(3): 491-502. |
| [3] | 由玉婉,张雨,孙嘉毅,张蔚. ‘月月粉’月季NAC家族全基因组鉴定及皮刺发育相关成员的筛选[J]. 中国农业科学, 2022, 55(24): 4895-4911. |
| [4] | 张琦,段玉,苏越,蒋琪琪,王春庆,宾羽,宋震. 基于柑橘叶斑驳病毒的表达载体构建及应用[J]. 中国农业科学, 2022, 55(22): 4398-4407. |
| [5] | 刘鑫,张亚红,袁苗,党仕卓,周娟. ‘红地球’葡萄花芽分化过程中的转录组分析[J]. 中国农业科学, 2022, 55(20): 4020-4035. |
| [6] | 肖桂华,文康,韩健,郝晨星,叶蓉春,朱亦赤,萧顺元,邓子牛,马先锋. 钙对枳生长发育及柑橘溃疡病抗性的影响[J]. 中国农业科学, 2022, 55(19): 3767-3778. |
| [7] | 储宝华,曹富国,卞宁宁,钱谦,李中兴,李雪薇,刘泽远,马锋旺,管清美. 84个苹果栽培品种对斑点落叶病的抗性评价和全基因组关联分析[J]. 中国农业科学, 2022, 55(18): 3613-3628. |
| [8] | 李依镁,王娇,王萍,师恺. 番茄糖转运蛋白SlSTP2在防御细菌性叶斑病中的功能[J]. 中国农业科学, 2022, 55(16): 3144-3154. |
| [9] | 方瀚墨,胡璋健,马巧梅,丁淑婷,王萍,王安然,师恺. 番茄SlβCA3在防御丁香假单胞菌番茄致病变种中的功能[J]. 中国农业科学, 2022, 55(14): 2740-2751. |
| [10] | 方桃红,张敏,马春花,郑晓晨,谭文静,田冉,燕琼,周新力,李鑫,杨随庄,黄可兵,王建锋,韩德俊,王晓杰,康振生. 小麦抗条锈基因Yr52在品种改良中的应用[J]. 中国农业科学, 2022, 55(11): 2077-2091. |
| [11] | 丁茜,赵凯茜,王跃进. 中国野生毛葡萄芪合酶基因表达及对葡萄抗白粉病的影响[J]. 中国农业科学, 2021, 54(2): 310-323. |
| [12] | 张婧芸,刘语诺,王兆昊,彭爱红,陈善春,何永睿. 转CiNPR4基因柑橘抗溃疡病的机制解析[J]. 中国农业科学, 2021, 54(18): 3871-3880. |
| [13] | 张勇,阎俊,肖永贵,郝元峰,张艳,徐开杰,曹双河,田宇兵,李思敏,闫俊良,张赵星,陈新民,王德森,夏先春,何中虎. 中麦895高产稳产优质特性遗传解析[J]. 中国农业科学, 2021, 54(15): 3158-3167. |
| [14] | 赵子麒,赵雅琪,林昌朋,赵永泽,余宇潇,孟庆立,曾广莹,薛吉全,杨琴. 48份玉米自交系抗病性的精准鉴定[J]. 中国农业科学, 2021, 54(12): 2510-2522. |
| [15] | 彭蕴,雷天刚,邹修平,张靖芸,张庆雯,姚家欢,何永睿,李强,陈善春. 柑橘溃疡病抗性SNP验证及其相关钙依赖性蛋白激酶基因诱导表达[J]. 中国农业科学, 2020, 53(9): 1820-1829. |
|
||