













中国农业科学 ›› 2021, Vol. 54 ›› Issue (2): 310-323.doi: 10.3864/j.issn.0578-1752.2021.02.007
收稿日期:2020-04-20
接受日期:2020-06-05
出版日期:2021-01-16
发布日期:2021-02-03
通讯作者:
王跃进
作者简介:丁茜,E-mail: 基金资助:
DING Xi( ),ZHAO KaiXi,WANG YueJin(
),ZHAO KaiXi,WANG YueJin( )
)
Received:2020-04-20
Accepted:2020-06-05
Online:2021-01-16
Published:2021-02-03
Contact:
YueJin WANG
摘要:
【目的】克隆中国野生毛葡萄(Vitis quinquangularis)‘丹凤-2’芪合酶(stilbene synthase)基因(STS)并研究其功能,为提高欧洲葡萄(V. vinifera)的白粉病抗性及品质提供依据。【方法】利用同源克隆法获得中国野生毛葡萄‘丹凤-2’芪合酶基因VqSTS9和VqSTS21,构建植物过表达载体;用无核白单芽茎段诱导出分生愈伤组织,作为农杆菌介导法遗传转化的受体材料,获得抗性植株,经过不同水平检测,确定转基因植株;对野生型和转基因植株叶片人工接种葡萄白粉病菌(Uncinula necator),通过显微技术观察叶片受白粉病菌侵染后的情况,比较两者对白粉病的抗性;利用实时荧光定量PCR(qRT-PCR)分析野生型和转基因植株在自然条件和接种白粉病菌后STS及其相关基因的表达,用高效液相色谱法(HPLC)检测转基因植株中芪类物质的种类与含量。【结果】同源序列克隆得到VqSTS9(JQ868689)与VqSTS21(JQ868677)的cDNA序列,长度为1 179 bp。经PCR和Western blot检测,鉴定出过表达VqSTS9无核白植株4株和过表达VqSTS21无核白植株3株。显微观察发现,与野生型植株相比,转VqSTS9 和VqSTS21植株叶片上的菌丝生长较慢,表现出对白粉病的抗性。qRT-PCR结果表明,自然生长条件下,与野生型植株相比,转VqSTS9和VqSTS21植株STS的表达量提高,STS上游苯丙氨酸裂解酶基因(PAL)、下游白藜芦醇糖基转移酶基因(RSGT)、转录因子基因(MYB14、MYB15)的表达量均不同程度上升,而查尔酮合成酶基因(CHS)表达量降低;人工接种白粉病菌后,与野生型植株相比,转基因植株STS表达量显著上调。高效液相色谱分析表明,自然条件下,芪类物质主要以反式云杉新苷形式存在,转基因植株芪类物质的含量高于野生型植株;在接种白粉病菌诱导表达后,除了反式云杉新苷,还产生了反式白藜芦醇和葡萄素,即转基因植株体内芪类物质的种类和含量均有所增加。【结论】将VqSTS9、VqSTS21转入无核白后,转基因植株STS的表达量增高,芪类物质的含量与种类增加,并抑制白粉病菌的生长。因此,中国野生毛葡萄‘丹凤-2’携带的VqSTS9和VqSTS21能够增强欧洲葡萄对白粉病的抗性,‘丹凤-2’可用作葡萄抗病性育种的种质资源。
丁茜,赵凯茜,王跃进. 中国野生毛葡萄芪合酶基因表达及对葡萄抗白粉病的影响[J]. 中国农业科学, 2021, 54(2): 310-323.
DING Xi,ZHAO KaiXi,WANG YueJin. Expression of Stilbene Synthase Genes from Chinese Wild Vitis quinquangularis and Its Effect on Resistance of Grape to Powdery Mildew[J]. Scientia Agricultura Sinica, 2021, 54(2): 310-323.
表1
本研究所用引物"
| 基因 Gene | 上游引物 Forward primer | 下游引物 Reverse primer | 目标大小 Target size (bp) |
|---|---|---|---|
| VqSTS9 (vector construction, PCR detection) | F: CGGGATCCATGGCTTCAGTCGAGGAA TTTAGAAACG | R: GCGTCGACATTTGTAACCGTAGGAAT GCTATGCAGC | 1179 |
| VqSTS21 (vector construction, PCR detection) | F: CGGGATCCATGGCTTCAGTCGAGGAA ATTAGAAACG | R: GCGTCGACATTTGTAACCATAGGAAT GCTATGCAACAC | 1179 |
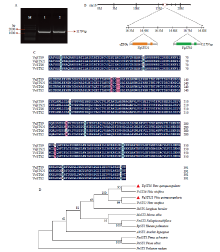
图2
VqSTS9、VqSTS21的克隆、序列与聚类分析 A:VqSTS9、VqSTS21目的基因克隆VqSTS9 and VqSTS21 gene cloning;B:VqSTS9、VqSTS21的染色体定位Chromosome localization of VqSTS9, VqSTS21;C:VqSTS9、VqSTS21与欧洲葡萄氨基酸序列比对Amino acid sequence alignment of VqSTS9, VqSTS21 and STS from V. vinifera;D:VqSTS9、VqSTS21与不同物种STS氨基酸序列聚类分析,包括欧洲葡萄、松叶兰、樟子松、云杉、高粱、花生、桑、何首乌、大黄Cluster analysis of amino acid sequence of VqSTS9, VqSTS21 and STS from V. vinifera, Psilotum nudum, Pinus sylvestris, Picea abies, Sorghum bicolor, Arachis hypogaea, Morus alba, Fallopia multiflora, Rheum palmatum。登录号The accession number:VqSTS9(AFM56666.1)、VqSTS21(AFM56657.1)、VvSTS6(NP_001267934.1)、VvSTS2(XP_003634068.1)、PnSTS(BAA87924)、PsylSTS(CAA43165)、PaSTS(AEN84236.1)、SbSTS(AAL49965)、AhSTS(BAA78617)、MaSTS(ARM20004.1)、FmSTS(AFP97667.1)、RpSTS(AFX68803.1)"
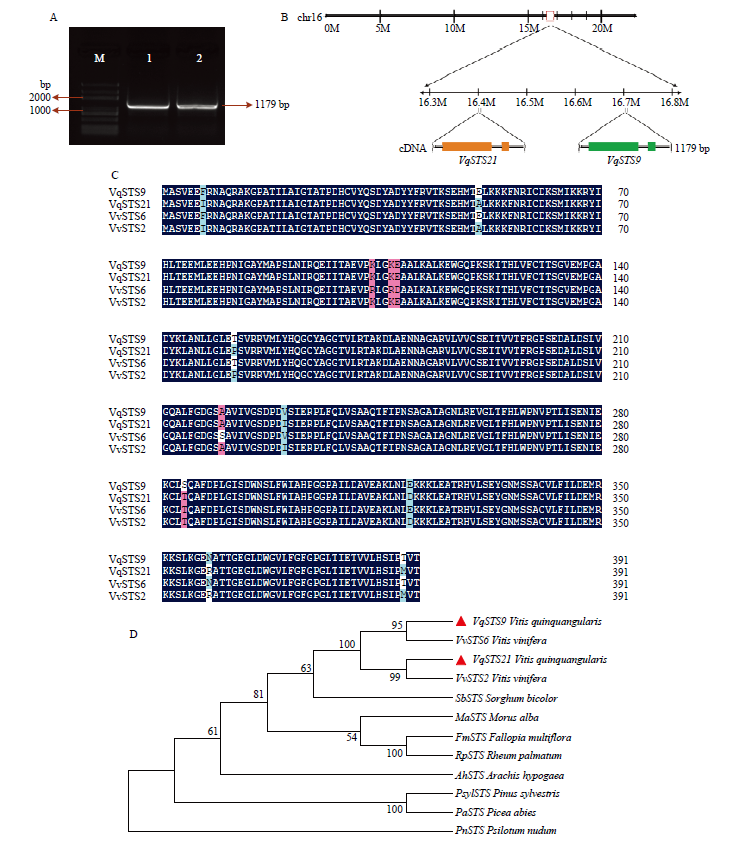
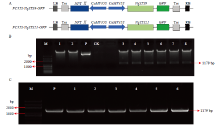
图3
VqSTS9、VqSTS21基因克隆与植物表达载体构建 A:两个表达载体示意图Schematic diagram of two expression vectors。B:VqSTS9、VqSTS21基因连接表达载体pCAMBIA2300的双酶切检测VqSTS9, VqSTS21 genetic connection expression vector pCAMBIA2300 double enzyme detection,M:DNA Marker;1:BamH Ι单酶切表达载体质粒The BamH Ι single enzyme expression vector plasmid;2:Sal Ι单酶切表达载体质粒The Sal Ι single enzyme expression vector plasmid;P:pCAMBIA2300的空载体质粒对照Empty vector pCAMBIA2300 as control;CK:空白对照Blank control (ddH2O);3—5:BamH Ι和Sal Ι双酶切植物表达载体 pCAMBIA35S::VqSTS9::GFP,3个重复Double enzyme of BamH Ι and Sal Ι plant expression vector pCAMBIA35S: :VqSTS9: :GFP, three repeats;6—8:BamH Ι和Sal Ι双酶切植物表达载体pCAMBIA35S::VqSTS21::GFP,3个重复Double enzyme of BamH Ι and Sal Ι plant expression vector pCAMBIA35S::VqSTS21::GFP, three repeats。C:过表达载体转化农杆菌的菌液PCR检测PCR detection of Agrobacterium-transformed by overexpression vector,M:DNA Marker;P:阳性质粒对照Positive plasmid control;1—3:农杆菌转化pCAMBIA35S::VqSTS9::GFP载体,3个重复Agrobacterium-transformed pCAMBIA35S::VqSTS9::GFP vector, three repeats;4—6:农杆菌转化pCAMBIA35S::VqSTS21::GFP载体, 3个重复Agrobacterium-transformed pCAMBIA35S::VqSTS21::GFP vector, three repeats"
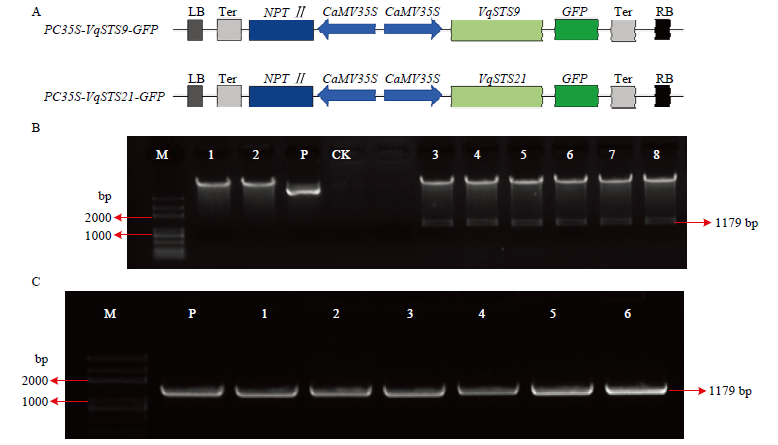
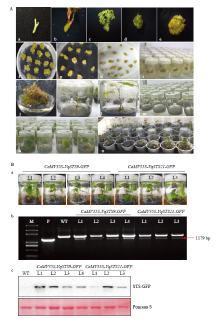
图4
无核白遗传转化过程(A)以及转基因植株的鉴定(B) A:无核白遗传转化过程The genetic transformation of Thompson Seedless,a:无核白分生愈伤组织Meristem callus;b:侵染后的愈伤组织与农杆菌共培养The infected callus was co-cultured with A. tumefaciens;c:转化后的愈伤组织筛选培养Callus screening culture after transformation;d:筛选60 d后的愈伤组织Callus screening culture after 60 days;e:愈伤组织诱导出抗性芽Callus induced resistant buds;f:抗性芽生根培养Rooting culture of resistant bud;g:抗性芽长成植株Resistant buds grow into plants;h:抗性植株的继代扩繁Secondary propagation of resistant plants;i:继代后长成植株Plant growth after succession;j:转基因植株移栽Transplanting of transgenic plants。B:转基因植株的鉴定Identification of transgenic plants,a:转基因植株Transgenic plants;b:PCR检测抗性植株PCR was used to detect resistant plants,M为Marker,P为质粒M was Marker, and P was plasmid;c:Western blot检测抗性植株Western blot was used to detect resistant plants"

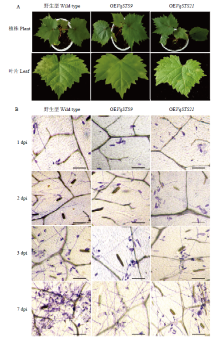
图6
转基因植株抗白粉病分析 A:用于接种白粉病菌的野生型无核白和转基因植株、与接种白粉病菌7 d的野生型和转基因的葡萄叶片Wildtype Thompson Seedless and transgenic plants used for U. necator induction and leaves with induction 7 dpi;B:显微观察葡萄叶片上白粉病菌的生长。标尺=100 μm The growth progress of U. necator on grape leaves was observed microscopically. Scale bar=100 μm"
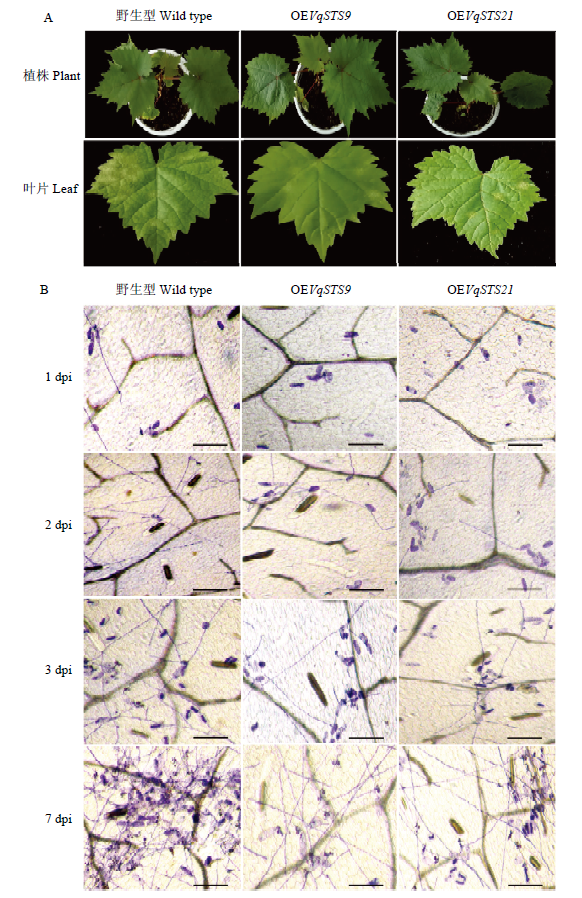
表2
每100个孢子萌发数、初级菌丝数、次级菌丝数和分生孢子梗数统计"
| 株系 Line | 1 dpi | 2 dpi | 3 dpi | 7 dpi | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 萌发数 Germination | 初级菌丝数 Primary hyphae | 次级菌丝数 Secondary hyphae | 萌发数 Germination | 初级菌丝数 Primary hyphae | 次级菌丝数 Secondary hyphae | 萌发数Germination | 初级菌丝数 Primary hyphae | 次级菌丝数 Secondary hyphae | 分生孢子 梗数 Conidiophore | |
| WT | 35.67±2.05a | 16.67±1.25a | 2.33±0.47a | 25.67±1.70a | 10.33±1.25d | 36.67±1.70a | 14.67±1.25d | 8.67±1.25c | 45.00±1.63a | 203.33±5.31a |
| OEVqSTS9-L1 | 21.33±2.36c | 5.67±1.25d | 0 | 14.67±1.25cd | 12.67±2.05cd | 5.33±1.25b | 22.33±2.49bc | 15.33±1.25a | 13.67±1.25c | 61.33±3.86e |
| OEVqSTS9-L2 | 16.67±1.70d | 8.00±2.45cd | 1.33±1.25a | 11.33±1.25d | 14.67±0.94bc | 6.67±1.70b | 21.67±1.70c | 11.67±1.25b | 16.33±0.47bc | 77.00±3.56d |
| OEVqSTS21-L2 | 26.67±2.05b | 10.67±1.70bc | 1.33±1.89a | 19.00±1.63b | 17.33±1.25ab | 7.33±1.25b | 27.33±1.25a | 14.33±1.70ab | 16.33±2.49bc | 87.33±3.68c |
| OEVqSTS21-L3 | 22.33±1.7bc | 13.00±0.82b | 0.67±0.94a | 17.33±1.70bc | 19.33±1.25a | 8.33±1.25b | 25.67±0.47ab | 16.00±0.82a | 18.00±1.63b | 124.67±4.11b |
| [1] | ALLEWELDT G, POSSINGHAM J V. Progress in grapevine breeding. Theoretical and Applied Genetics, 1988,75(5):669-673. |
| [2] |
QIU W, FEECHAN A, DRY I. Current understanding of grapevine defense mechanisms against the biotrophic fungus (Erysiphe necator), the causal agent of powdery mildew disease. Horticulture Research, 2015,2:15020.
doi: 10.1038/hortres.2015.20 pmid: 26504571 |
| [3] |
GLAWE D A. The powdery mildews: A review of the world’s most familiar (yet poorly known) plant pathogens. Annual Review of Phytopathology, 2008,46:27-51.
pmid: 18680422 |
| [4] |
TAKSONYI P, KOCSIS L, MÁTYAS K K, TALLER J. The effect of quinone outside inhibitor fungicides on powdery mildew in a grape vineyard in Hungary. Scientia Horticulturae, 2013,161:233-238.
doi: 10.1016/j.scienta.2013.06.031 |
| [5] |
BISSON L F, WATERHOUSE A L, EBELER S E, WALKER M A, LAPSLEY J T. The present and future of the international wine industry. Nature, 2002,418(6898):696-699.
pmid: 12167877 |
| [6] |
ZHOU Q, DAI L, CHENG S, HE J, WANG D, ZHANG J, WANG Y. A circulatory system useful both for long-term somatic embryogenesis and genetic transformation in Vitis vinifera L. cv. Thompson Seedless. Plant Cell, Tissue and Organ Culture, 2014,118:157-168.
doi: 10.1007/s11240-014-0471-y |
| [7] |
LANGCAKE P, PRYCE R J. A new class of phytoalexins from grapevines. Experientia, 1977,33(2):151-152.
doi: 10.1007/BF02124034 pmid: 844529 |
| [8] |
HAIN R, REIF H J, KRAUSE E, LANGEBARTELS R, KINDL H, VORNAM B, WIESE W, SCHMELZER E, SCHREIER P H, STÖCKER R H, STENZEL K. Disease resistance results from foreign phytoalexin expression in a novel plant. Nature, 1993,361(6408):153-156.
doi: 10.1038/361153a0 pmid: 8421520 |
| [9] | JANG M, CAI L, UDEANI G O, SLOWING K V, THOMAS C F, BEECHER C W, FONG H H, FARNSWORTH N R, KINGHORN A D, MEHTA R G, MOON R C, PEZZUTO J M. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science, 1997,275(5297):218-220. |
| [10] |
BAUR J A, SINCLAIR D A. Therapeutic potential of resveratrol: The in vivo evidence. Nature Reviews Drug Discovery, 2006,5:493-506.
doi: 10.1038/nrd2060 pmid: 16732220 |
| [11] |
SIREROL J A, RODRÍGUEZ M L, MENA S, ASENSI M A, ESTRELA J M, ORTEGA A L. Role of natural stilbenes in the prevention of cancer. Oxidative Medicine and Cellular Longevity, 2016,2016:3128951.
doi: 10.1155/2016/3128951 pmid: 26798416 |
| [12] |
CHENG S, XIE X, XU Y, ZHANG C, WANG X, ZHANG J, WANG Y. Genetic transformation of a fruit-specific, highly expressed stilbene synthase gene from Chinese wild Vitis quinquangularis. Planta, 2016,243(4):1041-1053.
doi: 10.1007/s00425-015-2459-1 pmid: 26781778 |
| [13] |
KOBAYASHI S, DING C K, NAKAMURA Y, NAKAJIMA I, MATSUMOTO R. Kiwifruits (Actinidia deliciosa) transformed with a Vitis stilbene synthase gene produce piceid (resveratrol-glucoside). Plant Cell Reports, 2000,19:904-910.
doi: 10.1007/s002990000203 pmid: 30754928 |
| [14] |
RÜHMANN S, TREUTTER D, FRITSCHE S, BRIVIBA K, SZANKOWSKI I. Piceid (resveratrol glucoside) synthesis in stilbene synthase transgenic apple fruit. Journal of Agricultural and Food Chemistry, 2006,54(13):4633-4640.
doi: 10.1021/jf060249l pmid: 16787008 |
| [15] |
YU C K, LAM C N, SPRINGOB K, SCHMIDT J, CHU I K, LO C. Constitutive accumulation of cis-piceid in transgenic Arabidopsis overexpressing a sorghum stilbene synthase gene. Plant and Cell Physiology, 2006,47(7):1017-1021.
doi: 10.1093/pcp/pcj061 pmid: 16731548 |
| [16] |
BAEK S H, SHIN W C, RYU H S, LEE D W, MOON E, SEO C S, HWANG E, LEE H S, AHN M H, JEON Y, et al. Creation of resveratrol-enriched rice for the treatment of metabolic syndrome and related diseases. PLoS ONE, 2013,8(3):e57930.
doi: 10.1371/journal.pone.0057930 pmid: 23483945 |
| [17] |
GIORCELLI A, SPARVOLI F, MATTIVI F, TAVA A, BALESTRAZZI A, VRHOVSEK U, CALLIGARI P, BOLLINI R, CONFALONIERI M. Expression of the stilbene synthase (StSy) gene from grapevine in transgenic white poplar results in high accumulation of the antioxidant resveratrol glucosides. Transgenic Research, 2004,13:203-214.
doi: 10.1023/b:trag.0000034658.64990.7f pmid: 15359598 |
| [18] |
HIPSKIND J D, PAIVA N L. Constitutive accumulation of a resveratrol-glucoside in transgenic alfalfa increases resistance to Phoma medicaginis. Molecular Plant-Microbe Interactions, 2000,13(5):551-562.
doi: 10.1094/MPMI.2000.13.5.551 pmid: 10796021 |
| [19] | DAI L, ZHOU Q, LI R, DU Y, HE J, WANG D, CHENG S, ZHANG J, WANG Y. Establishment of a picloram-induced somatic embryogenesis system in Vitis vinifera cv. chardonnay and genetic transformation of a stilbene synthase gene from wild-growing Vitis species. Plant Cell, Tissue and Organ Culture, 2015,121:397-412. |
| [20] | 刘梦琦, 吴凤颖, 王跃进. 中国野生毛葡萄芪合成酶基因表达与抗白粉病分析. 中国农业科学, 2019,52(14):2436-2449. |
| LIU M Q, WU F Y, WANG Y J. Expression of stilbene synthase gene and resistance to powdery mildew analysis of Chinese wild Vitis quinquangularis. Scientia Agricultura Sinica, 2019,52(14):2436-2449. (in Chinese) | |
| [21] | 吴凤颖, 刘梦琦, 王跃进. 中国野生毛葡萄芪合酶基因抗白粉病功能分析. 园艺学报, 2020,47(2):205-219. |
| WU F Y, LIU M Q, WANG Y J. Function analysis of stilbene synthase genes VqSTS12 and VqSTS25 of the resistance to powdery mildew in Vitis quinquangularis. Acta Horticulturae Sinica, 2020,47(2):205-219. (in Chinese) | |
| [22] | XIE X, AGOERO C B, WANG Y, WALKER M A. Genetic transformation of grape varieties and rootstocks via organogenesis. Plant Cell, Tissue and Organ Culture, 2016,126:541-552. |
| [23] |
SHI J, HE M, CAO J, WANG H, D1NG J, JIAO Y, LI R, HE J, WANG D, WANG Y. The comparative analysis of the potential relationship between resveratrol and stilbene synthase gene family in the development stages of grapes (Vitis quinquangularis and Vitis vinifera). Plant Physiology and Biochemistry, 2014,74:24-32.
doi: 10.1016/j.plaphy.2013.10.021 pmid: 24246671 |
| [24] | WANG Y, LIU Y, HE P, CHEN J, LAMIKANRAZ O, LU J. Evaluation of foliar resistance to Uncinula necator in Chinese wild Vitis spp. species. Vitis, 1995,34(3):159-164. |
| [25] | ZHOU Q, DU Y, CHENG S, LI R, ZHANG J, WANG Y. Resveratrol derivatives in four tissues of six wild Chinese grapevine species. New Zealand Journal of Crop and Horticultural Science, 2015,43(3):204-213. |
| [26] | LANGCAKE P, PRYCE R J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiological Plant Pathology, 1976,9:77-86. |
| [27] | LANGCAKE P, MCCARTHY W V. The relationship of resveratrol production to infection of grapevine leaves by Botrytis cinerea. Vitis, 1979,18:244-253. |
| [28] |
SCHÖPPNER A, KINDL H. Purification and properties of a stilbene synthase from induced cell suspension cultures of peanut. The Journal of Biological Chemistry, 1984,259(11):6806-6811.
pmid: 6427224 |
| [29] |
HAIN R, BIESELER B, KINDL H, SCHRÖDER G, STÖCKER R. Expression of a stilbene synthase gene in Nicotiana tabacum results in synthesis of the phytoalexin resveratrol. Plant Molecular Biology, 1990,15:325-335.
doi: 10.1007/BF00036918 pmid: 2103451 |
| [30] |
MALACARNE G, VRHOVSEK U, ZULINI L, CESTARO A, STEFANINI M, MATTIVI F, DELLEDONNE M, VELASCO R, MOSER C. Resistance to Plasmopara viticola in a grapevine segregating population is associated with stilbenoid accumulation and with specific host transcriptional responses. BMC Plant Biology, 2011,11:114.
pmid: 21838877 |
| [31] |
GIOVINAZZO G, D’AMICO L, PARADISO A, BOLLINI R, SPARVOLI F, DEGARA L. Antioxidant metabolite profiles in tomato fruit constitutively expressing the grapevine stilbene synthase gene. Plant Biotechnology Journal, 2005,3:57-69.
doi: 10.1111/j.1467-7652.2004.00099.x pmid: 17168899 |
| [32] |
RICHTER A, DE KATHEN A, DE LORENZO G, BRIVIBA K, HAIN R, RAMSAY G, JACOBSEN H J, KIESECKER H. Transgenic peas (Pisum sativum) expressing polygalacturonase inhibiting protein from raspberry (Rubus idaeus) and stilbene synthase from grape (Vitis vinifera). Plant Cell Reports, 2006,25:1166-1173.
pmid: 16802117 |
| [33] |
HÜSKEN A, BAUMERT A, MILKOWSKI C, BECKER H C, STRACK D, MÖLLERS C. Resveratrol glucoside (Piceid) synthesis in seeds of transgenic oilseed rape (Brassica napus L.). Theoretical and Applied Genetics, 2005,111:1553-1562.
pmid: 16160820 |
| [34] | LECKBAND G, LÖRZ H. Transformation and expression of a stilbene synthase gene of Vitis vinifera L. in barley and wheat for increased fungal resistance. Theoretical and Applied Genetics, 1998,96:1004-1012. |
| [35] |
SERAZETDINOVA L, OLDACH K H, LÖRZ H. Expression of transgenic stilbene synthases in wheat causes the accumulation of unknown stilbene derivatives with antifungal activity. Journal of Plant Physiology, 2005,162:985-1002.
pmid: 16173460 |
| [36] |
ZHU Y J, AGBAYANI R, JACKSON M C, TANG C S, MOORE P H. Expression of the grapevine stilbene synthase gene VST1 in papaya provides increased resistance against diseases caused by Phytophthora palmivora. Planta, 2004,220(2):241-250.
doi: 10.1007/s00425-004-1343-1 pmid: 15309535 |
| [37] | PEZET R, GINDRO K, VIRET O, SPRING J L. Glycosylation and oxidative dimerization of resveratrol are respectively associated to sensitivity and resistance of grapevine cultivars to downy mildew. Physiological and Molecular Plant Pathology, 2004,65(6):297-303. |
| [38] | NICOTRA S, CRAMAROSSA M R, MUCCI A, PAGNONI U M, RIVA S, FORTI L. Biotransformation of resveratrol: Synthesis of trans-dehydrodimers catalyzed by laccases from Myceliophtora thermophyla and from Trametes pubescens. Tetrahedron, 2004,60(3):595-600. |
| [39] | GINDRO K, SPRING J L, PEZET R, RICHTER H, VIRET O. Histological and biochemical criteria for objective and early selection of grapevine cultivars resistant to Plasmopara viticola. Vitis, 2006,45(4):191-196. |
| [40] |
XU W, YU Y, ZHOU Q, DING J, DAI L, XIE X, XU Y, ZHANG C, WANG Y. Expression pattern, genomic structure, and promoter analysis of the gene encoding stilbene synthase from Chinese wild Vitis pseudoreticulata. Journal of Experimental Botany, 2011,62(8):2745-2761.
pmid: 21504880 |
| [41] | CHONG J, POUTARAUD A, HUGUENEY P. Metabolism and roles of stilbenes in plants. Plant Science, 2009,177(3):143-155. |
| [42] |
VANNOZZI A, DRY I B, FASOLI M, ZENONI S, LUCCHIN M. Genome-wide analysis of the grapevine stilbene synthase multigenic family: genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biology, 2012,12:130.
pmid: 22863370 |
| [43] |
ZHENG X, DENG W, LUO K, DUAN H, CHEN Y Q, MCAVOY R, SONG S Q, PEI Y, LI Y. The cauliflower mosaic virus (CaMV) 35S promoter sequence alters the level and patterns of activity of adjacent tissue- and organ-specific gene promoters. Plant Cell Reports, 2007,26(8):1195-1203.
doi: 10.1007/s00299-007-0307-x pmid: 17340093 |
| [44] |
FERRER J L, AUSTIN M B, STEWART C, NOEL J P. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiology and Biochemistry, 2008,46:356-370.
doi: 10.1016/j.plaphy.2007.12.009 pmid: 18272377 |
| [45] |
HÖLL J, VANNOZZI A, CZEMMEL S, D’ONOFRIO C, WALKER A R, RAUSCH T, LUCCHIN M, BOSS P K, DRY I B, BOGS J. The R2R3-MYB transcription factors MYB14 and MYB15 regulate stilbene biosynthesis in Vitis vinifera. The Plant Cell, 2013,25(10):4135-4149.
pmid: 24151295 |
| [46] | ALTAMURA M M, CERSOSIMO A, MAJOLI C, CRESPAN M. Histological study of embryogenesis and organogenesis from anthers of Vitis rupestris du Lot cultured in vitro. Protoplasma, 1992,171:134-141. |
| [1] | 赵海霞,肖欣,董玘鑫,吴花拉,李成磊,吴琦. 苦荞愈伤遗传转化体系的优化及用于FtCHS1的过表达分析[J]. 中国农业科学, 2022, 55(9): 1723-1734. |
| [2] | 蔡苇荻,张羽,刘海燕,郑恒彪,程涛,田永超,朱艳,曹卫星,姚霞. 基于成像高光谱的小麦冠层白粉病早期监测方法[J]. 中国农业科学, 2022, 55(6): 1110-1126. |
| [3] | 郭泽西,孙大运,曲俊杰,潘凤英,刘露露,尹玲. 查尔酮合成酶基因在葡萄抗灰霉病和霜霉病中的作用[J]. 中国农业科学, 2022, 55(6): 1139-1148. |
| [4] | 冯子恒,宋莉,张少华,井宇航,段剑钊,贺利,尹飞,冯伟. 基于无人机多光谱和热红外影像信息融合的小麦白粉病监测[J]. 中国农业科学, 2022, 55(5): 890-906. |
| [5] | 胡朝月, 王凤涛, 郎晓威, 冯晶, 李俊凯, 蔺瑞明, 姚小波. 小麦抗条锈病基因对中国条锈菌主要流行小种的抗性分析[J]. 中国农业科学, 2022, 55(3): 491-502. |
| [6] | 张洁,姜长岳,王跃进. 中国野生毛葡萄转录因子VqWRKY6与VqbZIP1互作调控抗白粉病功能分析[J]. 中国农业科学, 2022, 55(23): 4626-4639. |
| [7] | 张琦,段玉,苏越,蒋琪琪,王春庆,宾羽,宋震. 基于柑橘叶斑驳病毒的表达载体构建及应用[J]. 中国农业科学, 2022, 55(22): 4398-4407. |
| [8] | 康忱,赵雪芳,李亚栋,田哲娟,王鹏,吴志明. 黄瓜CC-NBS-LRR家族基因鉴定及在霜霉病和白粉病胁迫下的表达分析[J]. 中国农业科学, 2022, 55(19): 3751-3766. |
| [9] | 储宝华,曹富国,卞宁宁,钱谦,李中兴,李雪薇,刘泽远,马锋旺,管清美. 84个苹果栽培品种对斑点落叶病的抗性评价和全基因组关联分析[J]. 中国农业科学, 2022, 55(18): 3613-3628. |
| [10] | 李依镁,王娇,王萍,师恺. 番茄糖转运蛋白SlSTP2在防御细菌性叶斑病中的功能[J]. 中国农业科学, 2022, 55(16): 3144-3154. |
| [11] | 方瀚墨,胡璋健,马巧梅,丁淑婷,王萍,王安然,师恺. 番茄SlβCA3在防御丁香假单胞菌番茄致病变种中的功能[J]. 中国农业科学, 2022, 55(14): 2740-2751. |
| [12] | 方桃红,张敏,马春花,郑晓晨,谭文静,田冉,燕琼,周新力,李鑫,杨随庄,黄可兵,王建锋,韩德俊,王晓杰,康振生. 小麦抗条锈基因Yr52在品种改良中的应用[J]. 中国农业科学, 2022, 55(11): 2077-2091. |
| [13] | 沙仁和,兰黎明,王三红,罗昌国. 苹果转录因子MdWRKY40b抗白粉病的机理[J]. 中国农业科学, 2021, 54(24): 5220-5229. |
| [14] | 张勇,阎俊,肖永贵,郝元峰,张艳,徐开杰,曹双河,田宇兵,李思敏,闫俊良,张赵星,陈新民,王德森,夏先春,何中虎. 中麦895高产稳产优质特性遗传解析[J]. 中国农业科学, 2021, 54(15): 3158-3167. |
| [15] | 赵子麒,赵雅琪,林昌朋,赵永泽,余宇潇,孟庆立,曾广莹,薛吉全,杨琴. 48份玉米自交系抗病性的精准鉴定[J]. 中国农业科学, 2021, 54(12): 2510-2522. |
|
||