













中国农业科学 ›› 2019, Vol. 52 ›› Issue (23): 4274-4284.doi: 10.3864/j.issn.0578-1752.2019.23.008
收稿日期:2019-05-28
接受日期:2019-08-14
出版日期:2019-12-01
发布日期:2019-12-01
通讯作者:
钱伟
作者简介:远俊虎,E-mail:jhyuan1998@163.com|丁一娟,E-mail:dding1989@163.com
基金资助:
YUAN JunHu,DING YiJuan,YANG WenJing,YAN BaoQin,CHAI YaRu,MEI JiaQin,QIAN Wei( )
)
Received:2019-05-28
Accepted:2019-08-14
Online:2019-12-01
Published:2019-12-01
Contact:
Wei QIAN
摘要:
【目的】由核盘菌(Sclerotinia sclerotiorum)引起的菌核病是我国油菜种植上的主要问题,严重威胁着菜籽产量及品质。分泌性蛋白在病原菌致病过程中起着重要作用,核盘菌基因组中包含大量编码分泌性蛋白的基因,本研究旨在鉴定并筛选与致病性相关的分泌蛋白基因,揭示核盘菌的致病机理,为菌核病防控提供重要靶标。【方法】采用SMART软件对核盘菌在侵染抗病、感病甘蓝过程中差异表达明显的8个具有信号肽的候选基因进行蛋白质结构域的分析,随后将SMART分析得到的结构域分别于SCOP、Pfam、PDB数据库进行功能注释。利用基因特异性引物进行目的基因特异片段的PCR扩增,构建pTRV2-Gene和pTRV2-GFP载体。随后等量混合含有pTRV1及pTRV2-Gene,pTRV1及pTRV2-GFP载体的重悬菌液。室温静置3 h后,利用针筒浸润法将pTRV2-Gene载体及对照(TRV-GFP)侵染5—6周龄的本氏烟(Nicotiana benthamiana)。侵染植株于黑暗环境中培养48 h后,再置于正常光照条件的环境中生长7 d。将直径6 mm的核盘菌PDA菌丝块接种于侵染9 d后的烟草叶片叶腹的中央,其中带菌面紧贴叶片,随后将接种植株培养48 h后统计病斑面积。提取接种48 h后的病斑及病斑周围组织叶片(距腐烂组织边缘1 cm左右)的RNA,利用特异引物进行目的基因的qRT-PCR,计算目的基因在携带TRV-HIGS载体的烟草植株中的相对表达量。【结果】SMART及结构域注释预测这8个候选基因可能参与了蛋白、核酸或多糖的水解,影响植物的免疫反应,参与核盘菌对药物耐受性的调节及生物素合成。核盘菌接种携带这8个基因的TRV-HIGS载体及对照载体的烟草,48 h后对照植株的病斑面积平均为3.44 cm 2,除SS1G_07655外,其余7个候选基因的TRV-HIGS植株上的病斑面积相比对照植株均显著减小(P≤0.05),其病斑面积介于1.63—2.61 cm 2。qRT-PCR结果显示,这7个致病相关的候选基因在核盘菌侵染烟草过程中的基因表达水平均显著低于对照(P≤0.05)。【结论】利用TRV介导的HIGS技术成功地对核盘菌中8个未知功能的分泌蛋白基因进行了功能鉴定,筛选到7个可能与核盘菌致病性相关的基因,其中对核盘菌致病性影响最大的SS1G_03146预测可能参与核盘菌生物素合成,同时SS1G_04343及SS1G_11912预测可能参与影响植物的免疫反应。
远俊虎,丁一娟,杨文静,闫宝琴,柴亚茹,梅家琴,钱伟. 利用TRV-HIGS技术鉴定核盘菌致病相关的分泌蛋白基因[J]. 中国农业科学, 2019, 52(23): 4274-4284.
YUAN JunHu,DING YiJuan,YANG WenJing,YAN BaoQin,CHAI YaRu,MEI JiaQin,QIAN Wei. Identification of Genes Encoding Secretory Proteins Related to the Pathogenicity of Sclerotinia sclerotiorum Using TRV-HIGS[J]. Scientia Agricultura Sinica, 2019, 52(23): 4274-4284.
表1
本试验所用引物信息"
| 基因 Gene | 引物序列 Primer sequence (5′-3′) | 产物大小 Product size (bp) | 用途 Usage |
|---|---|---|---|
| SS1G_00263 | F: CCGGAATTCCGGCAGCGCCTCAAGCTCGACTCAAATC R: CGAGCTCGCTGACGGTAGCGGAACCAACAACGG qF: TCTTTGAGGATGGAACTTGGAC qR: AGCCTGGCAAGCATAATCG | 273 | 基因扩增Gene amplification qRT-PCR |
| SS1G_04945 | F: CCGGAATTCCGGTCTCCTTCCTTCTCGGC R: CGAGCTCGCTCGTAATCGGCACCAT qF: TCTCAACGGTGCTCTTTACTTC qR: TTGCTATCAGGGACCCATCC | 263 | 基因扩增Gene amplification qRT-PCR |
| SS1G_03181 | F: CCGGAATTCCGGTGAGCGATGCTACCAACAGCGCCTAC R: CGAGCTCGCACCAGCACTTTGGAGAGCACCGTAA qF: TGCCGATTACGAGGGAACA qR: TGGAAACATCGACGGTGAAG | 210 | 基因扩增Gene amplification qRT-PCR |
| SS1G_04343 | F: CCGGAATTCCGGTGGTCTTTACGCTGGGTATTTC R: CGAGCTCGCACCGTTGGTTGTGTTTTCATT qF: TGGTCTTTACGCTGGGTATTTC qR: CAGACACTTGCGAATGGAGC | 284 | 基因扩增Gene amplification qRT-PCR |
| SS1G_03146 | F: CCGGAATTCCGGACATTTCCTCTTGAACCATCCCGTA R: CGAGCTCGTTTATGACACCCTTGTTTCCAGCGA qF: GCACATTTCCTCTTGAACCATC qR: GCAGTGTCACTTCCCACCATT | 309 | 基因扩增Gene amplification qRT-PCR |
| SS1G_02250 | F: CCGGAATTCCGGTCACACTTTTGGCATT R: CGAGCTCGATCAGCACGTTTTTCT qF: AAGCCAACACCAACCTCATC qR: CACTGGAGCGTAGTTCTCGTAG | 272 | 基因扩增Gene amplification qRT-PCR |
| SS1G_11912 | F: CCGGAATTCCGGTCAGCAGCTCCACCTCCACCACC R: CGAGCTCGATTCGCCTTTCCAAAATCCGTAT qF: TTCCCGAAACCGTTCCTAGT qR: TCACCATTGCTACTGCCACTT | 257 | 基因扩增Gene amplification qRT-PCR |
| SS1G_07655 Sstub1 (内参基因Actin gene) | F: CCGGAATTCCGGGCTACTGTTCCTTTGGACTACGCT R: CGAGCTCGTTACTGAGGAGTGAGTCGTGTCGG qF: AATATGCCAGAGCCATCACA qR: CAGCGTAGTCCAAAGGAACAG qF: GTGAGGCTGAGGGCTGTGTGA qR: CCTTTGGCGATGGGACG | 322 | 基因扩增Gene amplification qRT-PCR qRT-PCR |
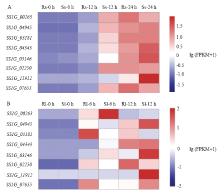
图1
候选基因在侵染抗、感病甘蓝过程中的表达热图 核盘菌候选基因在接种抗、感甘蓝茎秆(A)0、12、24 h和叶片(B) 0、6、12 h后的表达量Relative expression levels of candidate genes in the susceptible and resistant B. oleracea after inoculation with S. sclerotiorum in stem (A) post 0, 12, 24 h and leaf (B) post 0, 6, 12 h。Rs:抗病甘蓝材料的茎秆Resistant B. oleracea stem;Ss:感病甘蓝材料的茎秆Susceptible B. oleracea stem;Rl:抗病甘蓝材料的叶片Resistant B. oleracea leaf;Sl:感病甘蓝材料的叶片Susceptible B. oleracea leaf。以lg (FPKM+1)值绘制基因表达量的热图,红色表示高表达基因,蓝色表示低表达基因。颜色从红到蓝,表示lg (FPKM+1)从大到小。基因表达热图构建方法见文献[11] The lg (FPKM+1) value was used for the heatmap. Red indicated high expression genes and blue indicated low expression genes. The colors ranged from red to blue, indicating that lg (FPKM+1) from large to small. The heatmap was conducted according to reference [11]"
表2
候选基因的结构域功能分析"
| 基因 Gene | 结构域名称 Domain name | 结构域位置 Location (aa) | E期望值 E-value | 可能的功能或特性 Putative function or feature |
|---|---|---|---|---|
| SS1G_00263 | SCOP:d1hw1a2 | 14-79 | 0.77 | 调节药物的耐受性 Regulation of drug tolerance |
| SS1G_04945 | Pfam:Glyco_hydro_7 | 21-446 | 2.6e-200 | O型糖苷水解酶 O-glycoside hydrolase |
| PDB:3PL3|A | 19-446 | 0 | 纤维二糖水解酶 Cellobiohydrolase | |
| SCOP:d1gpia_ | 19-450 | 0 | 伴刀豆球蛋白A样凝集素/葡聚糖酶 Concanavalin A/Glucanase | |
| SS1G_03181 | Pfam:ASP | 91-399 | 3.2e-69 | 天冬氨酰蛋白酶(酸性蛋白酶)Aspartyl proteases (acid proteases) |
| SCOP:d1bxoa_ | 79-399 | 1e-81 | 酸性蛋白酶 Acid proteases | |
| PDB:3APP|A | 83-398 | 1e-90 | 酸性蛋白酶 Acid proteases | |
| SS1G_04343 | SCOP:d1gh8a_ | 339-377 | 0.39 | 翻译延长因子 Translation elongation factor |
| SCOP:d1fuia2 | 411-457 | 0.68 | 异构酶 Isomerase | |
| SS1G_03146 | SCOP:d1h4vb2 | 18-62 | 1.8 | 生物素合成酶 Biotin synthetase |
| SS1G_02250 | SCOP:d1ed8a_ | 43-90 | 5.7 | 碱性磷酸酶 Alkaline phosphatase |
| SCOP:d1dofa_ | 215-241 | 0.86 | L型天冬氨酸 L-aspartic acid | |
| SS1G_11912 | Pfam:NPP1 | 52-242 | 4.5e-61 | 坏死诱导蛋白 Necrosis inducing protein |
| PDB:3GNZ|P | 32-242 | 4e-65 | 毒素 Toxin | |
| SCOP:d1fuia2 | 188-240 | 0.58 | 异构酶 Isomerase | |
| SS1G_07655 | Pro-kuma_activ | 35-181 | 3.25e-19 | 肽酶 Peptidase |
| PDB:3EDY|A | 35-581 | 2e-67 | 肽酶 Peptidase | |
| SCOP:d1gt91_ | 36-581 | 9e-19 | 枯草杆菌蛋白酶类 Subtilisins |
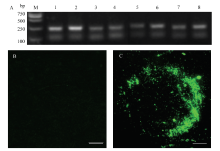
图3
TRV-HIGS转化烟草植株的鉴定 A:注射含有TRV-Gene重组质粒9 d后的烟草植株的PCR鉴定The identification of N. benthamiana at 9 days post TRV-Gene inoculation. M: Marker; 1: SS1G_00263; 2: SS1G_04945; 3: SS1G_03181; 4: SS1G_04343; 5: SS1G_03146; 6: SS1G_02250; 7: SS1G_11912; 8: SS1G_07655;B:注射不含重组质粒的重悬液9 d后的烟草植株的GFP信号观察GFP signal observation of N. benthamiana at 9 days after suspension without recombinant vectors inoculation;C:注射TRV-GFP载体9 d后的烟草植株的GFP信号观察GFP signal observation of N. benthamiana at 9 days post TRV-GFP inoculation。标尺Bar = 30 μm"
| [1] |
BOLAND G J, HALL R . Index of plant hosts of Sclerotinia sclerotiorum. Canadian Journal of Plant Pathology, 1994,16(2):93-108.
doi: 10.1094/PDIS-06-19-1147-RE pmid: 31746694 |
| [2] |
SEIFBARGHI S, BORHAN M H, WEI Y, COUTU C, ROBINSON S J, HEGEDUS D D . Changes in the Sclerotinia sclerotiorum transcriptome during infection of Brassica napus. BMC Genomics, 2017,18:266.
doi: 10.1186/s12864-017-3642-5 pmid: 28356071 |
| [3] |
YANG G, TANG L, GONG Y, XIE J, FU Y, JIANG D, LI G, COLLINGE D B, CHEN W, CHENG J . A cerato-platanin protein SsCP1 targets plant PR1 and contributes to virulence of Sclerotinia sclerotiorum. New Phytologist, 2018,217(2):739-755.
doi: 10.1111/nph.14842 pmid: 29076546 |
| [4] |
LYU X, SHEN C, FU Y, XIE J, JIANG D, LI G, CHENG J . A small secreted virulence-related protein is essential for the necrotrophic interactions of Sclerotinia sclerotiorum with its host plants. PLoS Pathogens, 2016,12(2):e1005435.
doi: 10.1371/journal.ppat.1005435 pmid: 26828434 |
| [5] |
ZHU W, WEI W, FU Y, CHENG J, XIE J, LI G, YI X, KANG Z, DICKMAN M B, JIANG D . A secretory protein of necrotrophic fungus Sclerotinia sclerotiorum that suppresses host resistance. PLoS ONE, 2013,8(1):e53901.
doi: 10.1371/journal.pone.0053901 pmid: 23342034 |
| [6] |
LYU X, SHEN C, FU Y, XIE J, JIANG D, LI G, CHENG J . Comparative genomic and transcriptional analyses of the carbohydrate- active enzymes and secretomes of phytopathogenic fungi reveal their significant roles during infection and development. Scientific Reports, 2015,5:15565.
doi: 10.1038/srep15565 pmid: 26531059 |
| [7] |
YU Y, XIAO J, ZHU W, YANG Y, MEI J, BI C, QIAN W, QING L, TAN W . Ss-Rhs1, a secretory Rhs repeat-containing protein, is required for the virulence of Sclerotinia sclerotiorum. Molecular Plant Pathology, 2017,18(8):1052-1061.
doi: 10.1111/mpp.12459 pmid: 27392818 |
| [8] |
XIAO X, XIE J, CHENG J, LI G, YI X, JIANG D, FU Y . Novel secretory protein Ss-Caf1 of the plant-pathogenic fungus Sclerotinia sclerotiorum is required for host penetration and normal sclerotial development. Molecular Plant-Microbe Interactions, 2014,27(1):40-55.
doi: 10.1094/MPMI-05-13-0145-R pmid: 24299212 |
| [9] |
DERBYSHIRE M, DENTON-GILES M, HEGEDUS D, SEIFBARGHI S, ROLLINS J, VAN KAN J, SEIDL M F, FAINO L, MBENGUE M, NAVAUD O, RAFFAELE S, HAMMOND-KOSACK K, HEARD S, OLIVER R . The complete genome sequence of the phytopathogenic fungus Sclerotinia sclerotiorum reveals insights into the genome architecture of broad host range pathogens. Genome Biology and Evolution, 2017,9(3):593-618.
doi: 10.1093/gbe/evx030 pmid: 28204478 |
| [10] |
GUYON K, BALAGUÉ C, ROBY D, RAFFAELE S . Secretome analysis reveals effector candidates associated with broad host range necrotrophy in the fungal plant pathogen Sclerotinia sclerotiorum. BMC Genomics, 2014,15:336.
doi: 10.1186/1471-2164-15-336 pmid: 24886033 |
| [11] |
DING Y, MEI J, CHAI Y, YU Y, SHAO C, WU Q, DISI J O, LI Y, WAN H, QIAN W . Simultaneous transcriptome analysis of host and pathogen highlights the interaction between Brassica oleracea and Sclerotinia sclerotiorum. Phytopathology, 2019,109(4):542-550.
doi: 10.1094/PHYTO-06-18-0204-R pmid: 30265202 |
| [12] |
KRAUSE C, RICHTER S, KNӦLL C, JÜRGENS G . Plant secretome—from cellular process to biological activity. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 2013,1834(11):2429-2441.
doi: 10.1016/j.bbapap.2013.03.024 pmid: 23557863 |
| [13] |
KUMAGAI M H, DONSON J, DELLA-CIOPPA G, HARVEY D, HANLEY K, GRILL L K . Cytoplasmic inhibition of carotenoid biosynthesis with virus-derived RNA. Proceedings of the National Academy of Sciences of the United States of America, 1995,92(5):1679-1683.
doi: 10.1073/pnas.92.5.1679 pmid: 7878039 |
| [14] |
BURCH-SMITH T M, ANDERSON J C, MAETIN G B, DINESH- KUMAR S P . Application and advantages of virus-induced gene silencing for gene function studies in plants. The Plant Journal, 2004,39(5):734-746.
doi: 10.1111/j.1365-313X.2004.02158.x pmid: 15315635 |
| [15] |
ROBERTSON D . VIGS vectors for gene silencing: Many targets, many tools. Annual Review of Plant Biology, 2004,55:495-519.
doi: 10.1146/annurev.arplant.55.031903.141803 pmid: 15377229 |
| [16] |
RUIZ M T, VOINNET O, BAULCOMBE D C . Initiation and maintenance of virus-induced gene silencing. The Plant Cell, 1998,10(6):937-946.
doi: 10.1105/tpc.10.6.937 pmid: 9634582 |
| [17] |
KJEMTRUP S, SAMPSON K S, PEELE C G, NGUYEN L V, CONKLING M A, THOMPSON W F, ROBERTSON D . Gene silencing from plant DNA carried by a Germinivirus. The Plant Journal, 1998,14(1):91-100.
doi: 10.1046/j.1365-313X.1998.00101.x pmid: 15494056 |
| [18] |
SONG Y, THOMMA B P . Host-induced gene silencing compromises Verticillium wilt in tomato and Arabidopsis. Molecular Plant Pathology, 2018,19(1):77-89.
doi: 10.1111/mpp.12500 pmid: 27749994 |
| [19] |
PANWAR V, MCCALLUM B, BAKKEREN G . Host-induced gene silencing of wheat leaf rust fungus Puccinia triticina pathogenicity genes mediated by the Barley stripe mosaic virus. Plant Molecular Biology, 2013,81(6):595-608.
doi: 10.1007/s11103-013-0022-7 |
| [20] |
田焕焕, 覃瑞, 刘虹, 刘清云, 李刚 . 病毒诱导基因沉默(VIGS)在禾本科植物中的研究进展. 植物学研究, 2014,3:91-104.
doi: 10.12677/BR.2014.33014 |
|
TIAN H H, QIN R, LIU H, LIU Q Y, LI G . Progress of virus induced gene silence (VIGS) system in the studies of Gramineae plant. Botanical Research, 2014,3:91-104. (in Chinese)
doi: 10.12677/BR.2014.33014 |
|
| [21] |
NOWARA D, GAY A, LACOMME C, SHAW J, RIDOUT C, DOUCHKOV D, HENSEL G, KUMLEHN J, SCHWEIZER P . HIGS: Host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. The Plant Cell, 2010,22(9):3130-3141.
doi: 10.1105/tpc.110.077040 pmid: 20884801 |
| [22] |
QI T, ZHU X, TAN C, LIU P, GUO J, KANG Z, GUO J . Host-induced gene silencing of an important pathogenicity factor PsCPK1 in Puccinia striiformis f. sp. tritici enhances resistance of wheat to stripe rust. Plant Biotechnology Journal, 2018,16(3):797-807.
doi: 10.1111/pbi.12829 pmid: 28881438 |
| [23] |
XU J, WANG X, LI Y, ZENG J, WANG G, DENG C, GUO W . Host-induced gene silencing of a regulator of G protein signalling gene ( VdRGS1) confers resistance to Verticillium wilt in cotton. Plant Biotechnology Journal, 2018,16(9):1629-1643.
doi: 10.1111/pbi.12900 pmid: 29431919 |
| [24] |
赵玉兰, 苏晓峰, 程红梅 . 利用寄主诱导的基因沉默技术验证大丽轮枝菌糖代谢相关基因的致病力. 中国农业科学, 2015,48(7):1321-1329.
doi: 10.3864/j.issn.0578-1752.2015.07.07 |
|
ZHAO Y L, SU X F, CHENG H M . Verification of Verticillium dahliae pathogenicity of glycometabolism related genes by using host-induced gene silencing method. Scientia Agricultura Sinica, 2015,48(7):1321-1329. (in Chinese)
doi: 10.3864/j.issn.0578-1752.2015.07.07 |
|
| [25] |
ANDRADE C M, TINOCO M L, RIETH A F, MAIA F C, ARAGᾸO F J . Host-induced gene silencing in the necrotrophic fungal pathogen Sclerotinia sclerotiorum. Plant Pathology, 2016,65(4):626-632.
doi: 10.3390/ijms19041138 pmid: 29642627 |
| [26] |
LIU E W, PAGE J E . Optimized cDNA libraries for virus-induced gene silencing (VIGS) using tobacco rattle virus. Plant Methods, 2008,4:5.
doi: 10.1186/1746-4811-4-5 pmid: 18211705 |
| [27] |
LIVAK K J, SCHMITTGEN T D . Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method . Methods, 2001,25(4):402-408.
doi: 10.1006/meth.2001.1262 pmid: 11846609 |
| [28] |
ZHENG Z, NONOMURA T, APPIANO M, PAVAN S, MATSUDA Y, TOYODA H, WOLTERS A M A, VISSER R G F, BAI Y, . Loss of function in Mlo orthologs reduces susceptibility of pepper and tomato to powdery mildew disease caused by Leveillula taurica. PLoS ONE, 2013,8(7):e70723.
doi: 10.1371/journal.pone.0070723 pmid: 23923019 |
| [29] |
吕琳慧, 徐幼平, 任至玄, 康冬, 王继鹏, 蔡新忠 . Ca2+信号通路对本氏烟叶位介导的核盘菌抗性的影响. 浙江大学学报(农业与生命科学版), 2014,40(6):605-610.
doi: 10.3785/j.issn.1008-9209.2014.03.131 |
|
LÜ L H, XU Y P, REN Z X, KANG D, WANG J P, CAI X Z . Effect of Ca2+ signaling pathway on leaf position-associated resistance to Sclerotinia sclerotiorum in Nicotiana benthamiana. Journal of Zhejiang University (Agriculture and Life Sciences), 2014,40(6):605-610. (in Chinese)
doi: 10.3785/j.issn.1008-9209.2014.03.131 |
|
| [30] | 王继鹏 . 菌龄对核盘菌致病性的影响及植物抗核盘菌分子机制[D]. 杭州: 浙江大学, 2015. |
| WANG J P . Molecular mechanisms underlying effect of mycelial age on pathogenicity of Sclerotinia sclerotiorum and plant resistance to this fungus[D]. Hangzhou: Zhejiang University, 2015. (in Chinese) | |
| [31] | 吴健, 周永明, 王幼平 . 油菜与核盘菌互作分子机理研究进展. 中国油料作物学报, 2018,40(5) : 721-729. |
| WU J, ZHOU Y M, WANG Y P . Research progress on molecular mechanisms of Brassica napus -Sclerotinia sclerotiorum interaction. Chinese Journal of Oil Crop Sciences, 2018,40(5):721-729. (in Chinese) | |
| [32] | 任晓梅 . 鸭疫里默氏杆菌生物素合成相关基因bioF生物学特性分析及应用[D]. 北京: 中国农业科学院, 2018. |
| REN X M . Biological characterization of biotin-synthesis associated bioF gene Riemerella anatipestifer and its application[D]. Beijing: Chinese Academy of Agricultural Sciences, 2018. (in Chinese) | |
| [33] |
HAYDON D J, GUEST J R . A new family of bacterial regulatory proteins. FEMS Microbiology Letters, 1991,79(2/3):291-295.
doi: 10.1016/j.biochi.2019.11.012 pmid: 31765672 |
| [34] | 曾洁 . 结核分枝杆菌GntR家族转录因子Rv1152在万古霉素耐受中的分子机理研究[D]. 重庆: 西南大学, 2016. |
| ZENG J . The underlying molecular mechanisms of Mycobacterium tuberculosis GntR family transcription factor Rv1152 in vancomycin resistance[D]. Chongqing: Southwest University, 2016. (in Chinese) | |
| [35] |
CHISHOLM S T, COAKER G, DAY B, STASKAWICZ B J . Host-microbe interactions: Shaping the evolution of the plant immune response. Cell, 2006,124(4):803-814.
doi: 10.1016/j.cell.2006.02.008 pmid: 16497589 |
| [36] |
KUNZE G, ZIPFEL C, ROBATZEK S, NIEHAUS K, BOLLER T, FELIX G . The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. The Plant Cell, 2004,16(12):3496-3507.
doi: 10.1105/tpc.104.026765 pmid: 15548740 |
| [1] | 邱一蕾,吴帆,张莉,李红亮. 亚致死剂量吡虫啉对中华蜜蜂神经代谢基因表达的影响[J]. 中国农业科学, 2022, 55(8): 1685-1694. |
| [2] | 杨时鳗, 许程志, 许榜丰, 吴运谱, 贾云慧, 乔传玲, 陈化兰. H1N1亚型猪流感病毒HA蛋白225位氨基酸对病毒致病性的影响[J]. 中国农业科学, 2022, 55(4): 816-824. |
| [3] | 裴悦宏,李凤巍,刘维娜,温玉霞,朱鑫,田绍锐,樊光进,马小舟,孙现超. 本氏烟半胱氨酸蛋白酶基因家族特征及其在TMV侵染中的功能[J]. 中国农业科学, 2022, 55(21): 4196-4210. |
| [4] | 温玉霞,张坚,王琴,王靖,裴悦宏,田绍锐,樊光进,马小舟,孙现超. 本氏烟NbMBF1c的克隆、表达及在TMV侵染过程中的功能[J]. 中国农业科学, 2022, 55(18): 3543-3555. |
| [5] | 李正刚,汤亚飞,佘小漫,于琳,蓝国兵,何自福. 侵染萝卜的油菜花叶病毒广东分离物分子特征及其致病性分析[J]. 中国农业科学, 2022, 55(14): 2752-2761. |
| [6] | 朱春艳,宋佳伟,白天亮,王娜,马帅国,普正菲,董艳,吕建东,李杰,田蓉蓉,罗成科,张银霞,马天利,李培富,田蕾. NaCl胁迫对不同耐盐性粳稻种质幼苗叶绿素荧光特性的影响[J]. 中国农业科学, 2022, 55(13): 2509-2525. |
| [7] | 李晓菁,张思雨,刘迪,袁晓伟,李兴盛,石延霞,谢学文,李磊,范腾飞,李宝聚,柴阿丽. 芸薹根肿菌活细胞PMAxx-qPCR快速定量检测方法的建立与应用[J]. 中国农业科学, 2022, 55(10): 1938-1948. |
| [8] | 许晨,王文静,曹珊,李如雪,张贝贝,孙爱清,张春庆. 花后DA-6处理调控小麦种子活力的机理[J]. 中国农业科学, 2021, 54(9): 1821-1834. |
| [9] | 李天聪,朱行,魏宁,龙凤,武建颖,张燕,董金皋,申珅,郝志敏. 玉米大斑病菌SC35同源基因表达规律与互作分析[J]. 中国农业科学, 2021, 54(4): 733-743. |
| [10] | 赵立群,邱艳红,张晓飞,刘慧,杨静静,张建,张海军,徐秀兰,温常龙. TaqMan探针法实时荧光定量PCR检测西瓜潜隐病毒[J]. 中国农业科学, 2021, 54(20): 4337-4347. |
| [11] | 张丽,汤亚飞,李正刚,于琳,蓝国兵,佘小漫,何自福. 侵染广东省葫芦科作物的中国南瓜曲叶病毒的分子特征[J]. 中国农业科学, 2021, 54(19): 4097-4109. |
| [12] | 郑信诗,尚鹏祥,李景远,丁新伦,吴祖建,张洁. 木尔坦棉花曲叶病毒“C4 ORF”编码蛋白对病毒致病性的影响[J]. 中国农业科学, 2021, 54(10): 2095-2104. |
| [13] | 常佳迎,刘树森,石洁,郭宁,张海剑,马红霞,杨春凤. 海南三亚和黄淮海地区玉米小斑病菌致病性及遗传多样性分析[J]. 中国农业科学, 2020, 53(6): 1154-1165. |
| [14] | 李正刚,农媛,汤亚飞,佘小漫,于琳,蓝国兵,邓铭光,何自福. 侵染广东连州葫芦的黄瓜绿斑驳花叶病毒的分子特征 及致病性分析[J]. 中国农业科学, 2020, 53(5): 955-964. |
| [15] | 柴亚茹,丁一娟,周思钰,杨文静,闫宝琴,远俊虎,钱伟. HIGS-SsCCS转基因拟南芥的菌核病抗性鉴定[J]. 中国农业科学, 2020, 53(4): 761-770. |
|
||