













中国农业科学 ›› 2020, Vol. 53 ›› Issue (12): 2371-2384.doi: 10.3864/j.issn.0578-1752.2020.12.006
齐悦1,吕峻元1,张悦1,韦杰1,张娜1,杨文香1( ),刘大群2(
),刘大群2( )
)
收稿日期:2019-12-26
出版日期:2020-06-16
发布日期:2020-06-25
通讯作者:
杨文香,刘大群
作者简介:齐悦,E-mail:908952718@qq.com。
基金资助:
QI Yue1,LÜ JunYuan1,ZHANG Yue1,WEI Jie1,ZHANG Na1,YANG WenXiang1( ),LIU DaQun2(
),LIU DaQun2( )
)
Received:2019-12-26
Online:2020-06-16
Published:2020-06-25
Contact:
WenXiang YANG,DaQun LIU
摘要:
【目的】由小麦叶锈菌(Puccinia triticina)引起的小麦叶锈病是影响小麦生产的主要病害之一,在小麦与叶锈菌互作的过程中病菌向寄主细胞分泌效应蛋白,以调控寄主防御反应、发挥毒性功能。开展对小麦叶锈菌效应蛋白的研究,探索小麦叶锈菌的致病机制,为病害的持续防控提供依据。【方法】以小麦叶锈菌13-5-72与感病品种Thatcher互作的cDNA为模板扩增效应蛋白Pt18906,通过SignalP 4.1、TargetP 1.1、TMHMM 2.0和EffectorP 2.0软件对Pt18906进行序列特征分析,利用在线软件Swiss-Model预测Pt18906的三级结构,利用在线软件SOPMA预测Pt18906的二级结构。采用实时荧光定量PCR对Pt18906的表达模式进行分析,借助于烟草的异源表达系统对Pt18906进行抑制Bax和INF1诱导的细胞程序性死亡(programmed cell death, PCD)能力验证,利用酵母系统验证Pt18906的信号肽是否具有分泌功能,采用氨基酸逐步缺失的方法缺失突变Pt18906,从而确定其功能毒性motif;通过在烟草中瞬时表达Pt18906-GFP融合蛋白,结合质壁分离技术分析Pt18906的亚细胞定位,得出效应蛋白的作用位点;利用瞬时表达技术在以Thatcher为背景的不含抗病基因和含有不同抗病基因的全套近等基因系上开展Pt18906无毒性功能分析;采用细菌三型分泌系统(Type Ⅲ secretion system)介导的瞬时转化分析Pt18906对寄主防御反应的调控。【结果】从小麦叶锈菌13-5-72与感病品种Thatcher互作6 d的转录组文库中获得一个在接种24 h后显著高表达的、基因全长序列672 bp、编码223个氨基酸的候选效应蛋白Pt18906,该效应蛋白缺乏已知的功能结构域和保守基序,工作环境偏碱性,在烟草细胞中瞬时表达Pt18906,Pt18906能够抑制Bax和INF1诱导的细胞程序性死亡,表明该效应蛋白具有毒性功能,并且通过构建缺失突变体明确其28—47位氨基酸对其毒性功能具有重要作用,该效应蛋白定位于细胞核和细胞质,表明其作用于细胞内。Pt18906在单基因系抗病品种TcLr27+31和TcLr42上能够引起过敏性坏死反应,表明该效应蛋白的无毒性,Pt18906能够引起TcLr27+31中胼胝质的积累和活性氧的迸发,胼胝质随注射时间的增加而逐渐积累,活性氧在注射后的10 min达到最高。【结论】位于28—47位的氨基酸决定Pt18906的毒性主要功能,Pt18906能激发小麦TcLr27+31双层防御反应。
齐悦,吕峻元,张悦,韦杰,张娜,杨文香,刘大群. 小麦叶锈菌效应蛋白Pt18906激发TcLr27+31的双层防御反应[J]. 中国农业科学, 2020, 53(12): 2371-2384.
QI Yue,LÜ JunYuan,ZHANG Yue,WEI Jie,ZHANG Na,YANG WenXiang,LIU DaQun. Puccinia triticina Effector Protein Pt18906 Triggered Two-Layer Defense Reaction in TcLr27+31[J]. Scientia Agricultura Sinica, 2020, 53(12): 2371-2384.
表1
本试验所用引物"
| 引物类型 Primer type | 引物名称 Primer name | 引物序列 Primer sequence (5′-3′) | 序列长度 Sequence length (bp) |
|---|---|---|---|
| Pt18906的ORF引物 Primer for Pt18906 ORF 信号肽分泌功能验证 Function verification of signal peptide secretion 异源瞬时表达 Heterogeneous transient expression 亚细胞定位 Subcellular localization 毒性结构域验证 Verification of virulent domain 细菌三型分泌系统 Bacterial type Ⅲ secretion system | ORFPt18906-F ORFPt18906-R SPPt18906-F SPPt18906-R Pt18906-F ΔSPPt18906-F Pt18906-R LBA LBB DPt18906-F DPt18906-R qPt18906-F1 qPt18906-R1 qPt18906-R2 qPt18906-R3 qPt18906-R4 PPt18906-F PPt18906-R | ATGTTTTCAGCAAGTTCAAT CTACTTACCCTTCTCCTTAG CCGGAATTCATGTTTTCAGCAAGTTCAAT CCGCTCGAGAAGCTCAACGGGTGGTAAGC TCCCCCGGGATGTTTTCAGCAAGTTCAAT TCCCCCGGGGCCGAAGTCCAACGACACGC GCGTCGACCTACTTACCCTTCTCCTTAG CAATCACAGTGTTGGCTTGC GACCCTATGGGCTGTGTTG CCCATCGATGCCGAAGTCCAACGACACGC TCCCCCGGGCTTACCCTTCTCCTTAGGAT CCCATCGATGCTCCACTCAAAAACGGTGA TCCCCCGGGTTGGTTGTTAACGTCCTCAA TCCCCCGGGGACCGCCATGTATCCGGCTA TCCCCCGGGGGGGTCTATCTCTGTAGGAT TCCCCCGGGTATGCTCCCGTATTTGCATG CACCATGTTTTCAGCAAGTTCAAT CTACTTACCCTTCTCCTTAG | 20 20 29 29 29 29 28 21 20 29 29 29 29 29 29 29 24 20 |

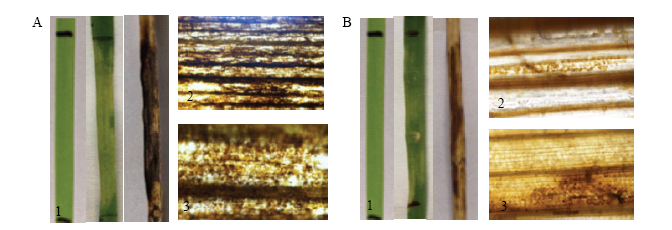
图7
在小麦中过表达Pt18906激发坏死反应 A:在TcLr27+31过表达Pt18906激发坏死Overexpression of Pt18906 in TcLr27+31 stimulates HR;B:在TcLr42过表达Pt18906激发坏死Overexpression of Pt18906 in TcLr42 stimulates HR 1:注射的表型结果Phenotypic results of injection;2:4倍荧光显微镜下的H2O2 The H2O2 under fluorescence microscope (4×);3:20倍荧光显微镜下的H2O2 The H2O2 under fluorescence microscope (20×)"
| [1] | RATTU A R, AHMAD I, FAYYAZ M, AKHTAR M A, IRFAN-UL-HAQUE , ZAKRIA M, AFZAL S N. Virulence analysis of Puccinia triticinia cause of leaf rust of wheat. Pakistan Journal of Botany, 2009,41(4):1957-1964. |
| [2] | ROELFS A P, SINGH R P, SAARI E E. Rust Disease of Wheat: Concepts and Methods of Disease Management. Mexico, DF: CMMYT, 1992: 7-14. |
| [3] | KOLMER J A. Genetics of resistance to wheat leaf rust. Annual Review of Phytopathology, 1996,34:435-455. |
| [4] |
RAMACHANDRAN S R, YIN C T, KUD J, TANAKA K, MAHONEY A K, XIAO F M, HULBERT S H. Effectors from wheat rust fungi suppress multiple plant defense responses. Phytopathology, 2017,107(1):75-83.
pmid: 27503371 |
| [5] | 刘冰玉, 蔡宝珊, 高成江. 泛素化调控抗病毒天然免疫的研究进展. 中国科学: 生命科学, 2018,48(11):1152-1161. |
| LIU B Y, CAI B S, GAO C J. Regulation of innate antiviral immunity by protein ubiquitination. Scientia Sinica Vitae, 2018,48(11):1152-1161. (in Chinese) | |
| [6] |
SCHORNACK S, BALLVORA A, GURLEBECK D, PEART J, GANAL M, BAKER B, BONAS U, LAHAYE T. The tomato resistance protein Bs4 is a predicted non-nuclear TIR-NB-LRR protein that mediates defense responses to severely truncated derivatives of AvrBs4 and overexpressed AvrBs3. The Plant Journal, 2004,37(1):46-60.
doi: 10.1046/j.1365-313x.2003.01937.x pmid: 14675431 |
| [7] |
THOMMA B P H J, NUMBERGER T, JOOSTEN M H A J. Of PAMPs and effectors: The Blurred PTI-ETI dichotomy. The Plant Cell, 2011,23(1):4-15.
pmid: 21278123 |
| [8] | 李伟兰, 戎伟, 何朝族. 一个推测的野油菜黄单胞菌Ⅲ型分泌效应子效应子基因XopXccP对寄主植物的致病性分析. 植物病理学报, 2014,44(2):173-179. |
| LI W L, RONG W, HE C Z. XopXccP, a putative type Ⅲ effector gene of Xanthomonas campestris pv. campestris, is required for pathogenicity on host plants. Acta Phytopathologica Sinica, 2014,44(2):173-179. (in Chinese) | |
| [9] |
CHASTAGNER P, ISRAEL A, BROU C. AIP4/Itch regulates notch receptor degradation in the absence of ligand. PLoS ONE, 2008,3(7):e2735.
pmid: 18628966 |
| [10] |
ONG L E, INNES R W. AvrB mutants lose both virulence and avirulence activities on soybean and Arabidopsis. Molecular Microbiology, 2006,60(4):951-962.
doi: 10.1111/j.1365-2958.2006.05162.x pmid: 16677306 |
| [11] | LI L, LI M, YU L, ZHOU Z, LIANG X, LIU Z, CAI G, GAO L, ZHANG X, WANG Y, CHEN S, ZHOU J M. The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host and Microbe, 2014,15(3):329-338. |
| [12] |
LU D, WU S, GAO X, ZHANG Y, SHAN L, HE P. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proceedings of the National Academy of Sciences of the United States of America, 2010,107(1):496-501.
doi: 10.1073/pnas.0909705107 pmid: 20018686 |
| [13] |
MONAGHAN J, MATSCHI S, SHORINOLA O, ROVENICH H, MATEI A, SEGONZAC C, MALINOVSKY F G, RATHJEN J P, MACLEAN D, ROMEIS T, ZIPFEL C. The calcium-dependent protein kinase CPK28 buffers plant immunity and regulates BIK1 turnover. Cell Host and Microbe, 2014,16(5):605-615.
doi: 10.1016/j.chom.2014.10.007 pmid: 25525792 |
| [14] | ZHANG J, LI W, XIANG T, LIU Z, LALUK K, DING X, ZOU Y, GAO M, ZHANG X, CHEN S, MENGISTE T, ZHANG Y, ZHOU J M. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host and Microbe, 2010,7(4):290-301. |
| [15] | 白志英, 王冬梅, 侯春燕, 刘娜, 韩胜芬, 马利华. 小麦叶锈菌侵染过程的显微和超微结构. 细胞生物学杂志, 2003,25(6):393-397. |
| BAI Z Y, WANG D M, HOU C Y, LIU N, HAN S F, MA L H. Microstructure and ultrastructure infected by wheat rust fungus. Chinese Journal of Cell Biology, 2003,25(6):393-397. (in Chinese) | |
| [16] |
SPERSCHNEIDER J, GARDINER D M, DODDS P N, TINI F, COVARELLI L, SINGH K B, MANNERS J M, TAYLOR J M. EffectorP: Predicting fungal effector proteins from secretomes using machine learning. New Phytologist, 2016,210(2):743-761.
doi: 10.1111/nph.13794 pmid: 26680733 |
| [17] |
TAKKEN F L, GOVERSE A. How to build a pathogen detector: Structural basis of NB-LRR function. Current Opinion in Plant Biology, 2012,15(4):375-384.
pmid: 22658703 |
| [18] |
JAROSE A M, BURDON J J. Host-pathogen interactions in natural populations of Linum marginale and Melampsora lini: II. Local and regional variation in patterns of resistance and racial structure. Evolution, 1991,45(7):1618-1627.
doi: 10.1111/j.1558-5646.1991.tb02667.x pmid: 28564135 |
| [19] |
DUPLESSIS S, CUOMO C A, LIN Y C, AERTS A, TISSERANT E, VENEAULT-FOURREY C, JOLY D L, HACQUARD S, AMSELEM J, CANTAREL B L,et al. Obligate biotrophy features unraveled by the genomic analysis of rust fungi. Proceedings of the National Academy of Sciences of the United States of America, 2011,108(22):9166-9171.
doi: 10.1073/pnas.1019315108 pmid: 21536894 |
| [20] | STASKAWICZ B J, MUDGETT M B, DANGL J L, GALAN J E. Common and contrasting themes of plant and animal diseases. Science, 2001,292(5525):2285-2289. |
| [21] |
CUI H, TSUDA K, PARKER J E. Effector-triggered immunity: From pathogen perception to robust defense. Annual Review of Plant Biology, 2015,66:487-511.
pmid: 25494461 |
| [22] |
BRUCE M, NEUGEBAUER K A, JOLY D L, MIGEON P, CUOMO C A, WANG S, AKHUNOV E, BAKKEREN G, KOLMER J A, FELLERS J P. Using transcription of six Puccinia triticina races to identify the effective secretome during infection of wheat. Frontiers in Plant Science, 2014,4: Article 520.
pmid: 24454317 |
| [23] |
HU Z, YAN C, LIU P, HUANG Z, MA R, ZHANG C, WANG R, ZHANG Y, MARTINON F, MIAO D, DENG H, WANG J, CHANG J, CHAI J. Crystal structure of NLRC4 reveals its autoinhibition mechanism. Science, 2013,341(6142):172-175.
doi: 10.1126/science.1236381 pmid: 23765277 |
| [24] |
CATANZARITI A M, DODDS P N, LAWRENCE G J, AYLIFFE M A, ELLIS J G. Haustorially expressed secreted proteins from flax rust are highly enriched for avirulence elicitors. The Plant Cell, 2006,18(1):243-256.
pmid: 16326930 |
| [25] | 杨作民, 解超杰, 孙其信. 后条中32时期我国小麦条锈抗源之现状. 作物学报, 2003,29(2):161-168. |
| YANG Z M, XIE C J, SUN Q X. Situation of the sources of stripe rust resistance of wheat in the post-CY32 era in China. Acta Agronomica Sinica, 2003,29(2):161-168. (in Chinese) | |
| [26] |
CUOMO C A, BAKKEREN G, KHALIL H B, PANWAR V, JOLY D, LINNING R, SAKTHIKUMAR S, SONG X, ADICONIS X, FAN L,et al. Comparative analysis highlights variable genome content of wheat rusts and divergence of the mating loci. G3: Genes, Genomes, Genetics, 2017,7(2):361-376.
doi: 10.1534/g3.116.032797 pmid: 27913634 |
| [27] |
CATANZARITI A M, DODDS P N, VE T, KOBE B, ELLIS J G, STASKAWICZL B J. The AvrM effector from flax rust has a structured c-terminal domain and interacts directly with the M resistance protein. Molecular Plant-Microbe Interactions, 2010,23(1):49-57.
doi: 10.1094/MPMI-23-1-0049 pmid: 19958138 |
| [28] |
DODDS P N, LAWRENCE G J, CATANZARITI A M, AYLIFFE M A, ELLIS J G. The Melampsora lini AvrL567 avirulence genes are expressed in haustoria and their products are recognized inside plant cells. The Plant Cell, 2004,16(3):755-768.
doi: 10.1105/tpc.020040 pmid: 14973158 |
| [29] |
GIRALDO M C, VALENT B. Filamentous plant pathogen effectors in action. Nature Reviews Microbiology, 2013,11:800-814.
doi: 10.1038/nrmicro3119 |
| [30] |
VANDER MERVE M M, KINNEAR M W, BARRETT L G, DODDS P N, ERICSON L, THRALL P H, BURDON J J. Positive selection in AvrP4 avirulence gene homologues across the genus Melampsora. Proceedings of the Royal Society B: Biological Sciences, 2009,276(1669):2913-2922.
doi: 10.1098/rspb.2009.0328 pmid: 19457888 |
| [31] |
ANDERSON C, KHAN M A, CATANZARITI A M, JACK C A, NEMRI A, LAWRENCE G J, UPADHYAYA N M, HARDHAM A R, ELLIS J G, DODDS P N, JONES D A. Genome analysis and avirulence gene cloning using a high-density RADseq linkage map of the flax rust fungus, Melampsora lini. BMC Genomics, 2016,17(1):667.
doi: 10.1186/s12864-016-3011-9 |
| [32] |
OH M, RHA G B, YOON J H, SUNWOO Y, HONG S H, PARK S D. RTP1, a rat homologue of adenovims E1A-associated Protein BS69, interacts with DNA topoisomerase II. Korean Journal of Biological Sciences, 2002,6(3):277-282.
doi: 10.1080/12265071.2002.9647663 |
| [33] |
PETER B, JOLY D L, DUPLEESSIS S. Effector proteins of rust fungi. Frontiers in Plant Science, 2014, 5: Article 416.
doi: 10.3389/fpls.2014.00416 pmid: 25191335 |
| [34] |
SALCEDO A, RUTTER W, WANG S, AKHUNOVA A, BOLUS S, CHAO S, ANDERSON N, DE SOTO M F, ROUSE M, SZABO L, BOWDEN R L, DUBCOVSKY J, AKHUNOVL E. Variation in the AvrSr35 gene determines Sr35 resistance against wheat stem rust race Ug99. Science, 2017,358(6370):1604-1606.
doi: 10.1126/science.aao7294 pmid: 29269474 |
| [35] |
CHEN J, UPADHYAYA N M, ORTIZ D, SPERSCHNEIDER J, LI F, BOUTON C, BREEN S, DONG C, XU B, ZHANG X X,et al. Loss of AvrSr50 by somatic exchange in stem rust leads to virulence for Sr50 resistance in wheat. Science, 2017,358(6370):1607-1610.
doi: 10.1126/science.aao4810 pmid: 29269475 |
| [36] | 汤春蕾. 条锈菌与小麦互作中效应蛋白及诱导寄主细胞坏死基因的鉴定与功能分析[D]. 杨凌: 西北农林科技大学, 2013. |
| TANG C L. Characterization and function analyses of host cell death inducing genes in wheat and Puccinia striiformis interactions[D]. Yangling: Northwest A&F University, 2013. (in Chinese) | |
| [37] | 宋平, 谭成龙, 郭嘉, 戚拓, 刘芃, 郭军. 小麦条锈菌效应蛋白基因PSTG_23616的时空表达特征分析. 西北农业学报, 2016,25(9):1279-1288. |
| SONG P, TAN C L, GUO J, QI T, LIU P, GUO J. Spatial and temporal expression pattern of effector protein gene PSTG_23616 in Puccinia striiformis f. sp. tritici. Acta Agriculturae Boreali-Occidentalis Sinica, 2016,25(9):1279-1288. (in Chinese) | |
| [38] |
CHENG Y L, WU K, YAO J N, LI S M, WANG X J, HUANG L L, KANG Z S. PSTha5a23, a candidate effector from the obligate biotrophic pathogen Puccinia striiformis f. sp. tritici, is involved in plant defense suppression and rust pathogenicity. Environmental Microbiology, 2017,19(5):1717-1729.
doi: 10.1111/1462-2920.13610 pmid: 27871149 |
| [39] |
CANTU D, SEGOVIA V, MACLEAN D, BAYLES R, CHEN X M, KAMOUN S, DUBCOVSKY J, SAUNDERS D G, UAUY C. Genome analyses of the wheat yellow (stripe) rust pathogen Puccinia striiformis f. sp. tritici reveal polymorphic and haustorial expressed secreted proteins as candidate effectors. BMC Genomics, 2013,14:270.
doi: 10.1186/1471-2164-14-270 pmid: 23607900 |
| [40] |
LIU C H, PEDERSEN C, SCHULTZ-LARSEN T, AGUILAR G B, MADRIZ-ORDENANA K, HOVMOLLER M S, THORDAL-CHRISTENSEN H. The stripe rust fungal effector PEC6 suppresses pattern-triggered immunity in a host species-independent manner and interacts with adenosine kinases. New Phytologist, 2016, doi: 10.1111/nph.14034.
doi: 10.1111/nph.16741 pmid: 32542680 |
| [41] | 季森, 赵梦鑫, 徐静华, 汤春蕾, 康振生, 王晓杰. 小麦条锈菌效应蛋白HASP2抑制寄主免疫反应. 植物病理学报, 2019,49(3):326-333. |
| JI S, ZHAO M X, XU J H, TANG C L, KANG Z S, WANG X J. Wheat stripe rust effector HASP2 inhibits host immune response. Acta Phytopathologica Sinica, 2019,49(3):326-333. (in Chinese) | |
| [42] | 王力坤, 樊昕, 汤春蕾, 康振生, 王晓杰. 条锈菌效应子Pst30抑制植物的胼胝质和活性氧积累. 植物病理学报, 2020,50(2):155-163. |
| WANG L K, FAN X, TANG C L, KANG Z S, WANG X J. Effector Pst30 from Puccinia striiformis f. sp. tritici inhibits callose deposition and ROS accumulation in plant. Acta Phytopathologica Sinica, 2020,50(2):155-163. (in Chinese) | |
| [43] | 陈增菊, 王婷, 汤春蕾, 赵梦鑫, 康振生, 王晓杰. 小麦条锈菌效应蛋白Hasp58抑制植物免疫的功能分析. 麦类作物学报, 2019,39(2):239-246. |
| CHEN Z J, WANG T, TANG C L, ZHAO M X, KANG Z S, WANG X J. Functional analysis of Puccinia striiformis f. sp. tritici effector Hasp58 inhibits plant immunity. Journal of Triticeae Crops, 2019,39(2):239-246. (in Chinese) | |
| [44] |
QI T, GUO J, LIU P, HE F, WAN C, ISLAM M A, TYLER B M, KANG Z S, GUO J. Stripe rust effector PstGSRE1 disrupts nuclear localization of ROS-promoting transcription factor TaLOL2 to defeat ROS-induced defense in wheat. Molecular Plant, 2019,12(12):1624-1638.
doi: 10.1016/j.molp.2019.09.010 pmid: 31606466 |
| [45] |
XU Q, TANG C L, WANG X D, SUN S T, ZHAO J R, KANG Z S, WANG X J. An effector protein of the wheat stripe rust fungus targets chloroplasts and suppresses chloroplast function. Nature Communications, 2019,10:5517.
doi: 10.1038/s41467-019-13398-6 pmid: 31822676 |
| [46] |
YANG Q, HUAI B, LU Y, CAI K, GUO J, ZHU X, KANG Z H, GUO J. A stripe rust effector Pst18363 targets and stabilises TaNUDX23 that promotes stripe rust disease. New Phytologist, 2020,225(2):880-895.
doi: 10.1111/nph.16199 pmid: 31529497 |
| [47] |
SEGOVIA V, BRUCE M, JESSICA L, RUPP S, HUANG L, BAKKEREN G, TRICK H N, FELLERS J P. Two small secreted proteins from Puccinia triticina induce reduction of β-glucoronidase transient expression in wheat isolines containing Lr9, Lr24, and Lr26. Canadian Journal of Plant Pathology, 2016,38(1):91-102.
doi: 10.1080/07060661.2016.1150884 |
| [48] |
RIDOUT C J, SKAMNIOTI P, PORRITT O, SACRISTAN S, JONES J D G, BROWN J K M. Multiple avirulence paralogues in cereal powdery mildew fungi may contribute to parasite fitness and defeat of plant resistance. The Plant Cell, 2006,18(9):2402-2414.
doi: 10.1105/tpc.106.043307 pmid: 16905653 |
| [1] | 杨时鳗, 许程志, 许榜丰, 吴运谱, 贾云慧, 乔传玲, 陈化兰. H1N1亚型猪流感病毒HA蛋白225位氨基酸对病毒致病性的影响[J]. 中国农业科学, 2022, 55(4): 816-824. |
| [2] | 张晋龙,赵志博,刘巍,黄丽丽. 猕猴桃细菌性溃疡病菌T3SS关键效应蛋白基因致病功能[J]. 中国农业科学, 2022, 55(3): 503-513. |
| [3] | 赫磊,路凯,赵春芳,姚姝,周丽慧,赵凌,陈涛,朱镇,赵庆勇,梁文化,王才林,朱丽,张亚东. 水稻穗顶端退化突变体paa21的表型分析及基因克隆[J]. 中国农业科学, 2022, 55(24): 4781-4792. |
| [4] | 李正刚,汤亚飞,佘小漫,于琳,蓝国兵,何自福. 侵染萝卜的油菜花叶病毒广东分离物分子特征及其致病性分析[J]. 中国农业科学, 2022, 55(14): 2752-2761. |
| [5] | 胡荣蓉,丁世杰,郭赟,朱浩哲,陈益春,刘政,丁希,唐长波,周光宏. Trolox对猪肌肉干细胞增殖及分化的影响[J]. 中国农业科学, 2021, 54(24): 5290-5301. |
| [6] | 沙仁和,兰黎明,王三红,罗昌国. 苹果转录因子MdWRKY40b抗白粉病的机理[J]. 中国农业科学, 2021, 54(24): 5220-5229. |
| [7] | 张丽,汤亚飞,李正刚,于琳,蓝国兵,佘小漫,何自福. 侵染广东省葫芦科作物的中国南瓜曲叶病毒的分子特征[J]. 中国农业科学, 2021, 54(19): 4097-4109. |
| [8] | 郑信诗,尚鹏祥,李景远,丁新伦,吴祖建,张洁. 木尔坦棉花曲叶病毒“C4 ORF”编码蛋白对病毒致病性的影响[J]. 中国农业科学, 2021, 54(10): 2095-2104. |
| [9] | 常佳迎,刘树森,石洁,郭宁,张海剑,马红霞,杨春凤. 海南三亚和黄淮海地区玉米小斑病菌致病性及遗传多样性分析[J]. 中国农业科学, 2020, 53(6): 1154-1165. |
| [10] | 李正刚,农媛,汤亚飞,佘小漫,于琳,蓝国兵,邓铭光,何自福. 侵染广东连州葫芦的黄瓜绿斑驳花叶病毒的分子特征 及致病性分析[J]. 中国农业科学, 2020, 53(5): 955-964. |
| [11] | 李月月,周文鹏,路思倩,陈德荣,戴剑鸿,郭乔优,刘勇,李凡,谭冠林. 番茄斑驳花叶病毒在我国茄科作物上的发生及生物学特性[J]. 中国农业科学, 2020, 53(3): 539-550. |
| [12] | 顾超珩,闫燕燕,魏夕雅,史庆华,巩彪. 硫代腺苷甲硫氨酸促进番茄百菌清降解的生理机制[J]. 中国农业科学, 2019, 52(6): 1058-1065. |
| [13] | 王晓宁,梁欢,王帅,方文生,许景升,冯洁,徐进,曹坳程. 青枯菌铜抗性基因copA 的功能[J]. 中国农业科学, 2019, 52(5): 837-848. |
| [14] | 远俊虎,丁一娟,杨文静,闫宝琴,柴亚茹,梅家琴,钱伟. 利用TRV-HIGS技术鉴定核盘菌致病相关的分泌蛋白基因[J]. 中国农业科学, 2019, 52(23): 4274-4284. |
| [15] | 杜娇,王娅波,李雪华,黄志强,杨宇衡,毕朝位,余洋. 核盘菌γ-谷氨酰磷酸还原酶基因SsGPR1的功能分析[J]. 中国农业科学, 2018, 51(19): 3694-3703. |
|
||