













中国农业科学 ›› 2019, Vol. 52 ›› Issue (13): 2243-2255.doi: 10.3864/j.issn.0578-1752.2019.13.005
窦万福,祁静静,胡安华,陈善春,彭爱红,许兰珍,雷天刚,姚利晓,何永睿( ),李强(
),李强( )
)
收稿日期:2019-03-12
接受日期:2019-04-03
出版日期:2019-07-01
发布日期:2019-07-11
通讯作者:
何永睿,李强
作者简介:窦万福,E-mail:douwanfu@foxmail.com
基金资助:
DOU WanFu,QI JingJing,HU AnHua,CHEN ShanChun,PENG AiHong,XU LanZhen,LEI TianGang,YAO LiXiao,HE YongRui( ),LI Qiang(
),LI Qiang( )
)
Received:2019-03-12
Accepted:2019-04-03
Online:2019-07-01
Published:2019-07-11
Contact:
YongRui HE,Qiang LI
摘要:
【目的】CsBZIP40是一个与溃疡病抗性相关的转录因子,本研究旨在筛选转录因子CsBZIP40在响应柑橘溃疡病菌(Xanthomonas citri subsp. citri,Xcc)侵染过程中的互作蛋白,对CsBZIP40的互作网络进行分析,为柑橘抗溃疡病的分子育种提供理论依据。【方法】采用GST pull-down技术筛选柑橘在溃疡病菌侵染过程中CsBZIP40的互作蛋白。首先,构建带有GST标签的CsBZIP40蛋白融合表达载体,经IPTG(异丙基硫代半乳糖苷)诱导表达、纯化后获得GST-CsBZIP40融合蛋白作为诱饵蛋白;然后,将GST-CsBZIP40融合蛋白固定在谷胱甘肽亲和磁珠上,用固定在亲和磁珠的GST-CsBZIP40诱饵蛋白与接种溃疡病菌或LB培养基后柑橘叶片总蛋白进行孵育,与GST-CsBZIP40诱饵蛋白结合的蛋白复合物洗脱收集后进行SDS-PAGE凝胶电泳验证。将验证成功的样品洗脱液进行液相色谱串联质谱(LC-MS/MS)检测,鉴定出未侵染和侵染状态下CsBZIP40的互作蛋白,将检测到的蛋白利用甜橙基因组数据库进行注释,筛选出在溃疡病菌侵染过程中与CsBZIP40特异结合的蛋白,并进行GO、KEGG和互作网络分析。【结果】过表达CsBZIP40的转基因植株表型正常,与对照植株无明显差异。过表达CsBZIP40的转基因植株溃疡病抗性评价中病斑面积、病情指数均显著小于野生型植株,分别为野生型的45%和54%。以该转基因植株为材料成功提取出侵染溃疡病菌后和未接种溃疡病菌状态下的柑橘叶片总蛋白。成功构建出GST-CsBZIP40诱饵蛋白表达载体,诱导表达纯化出GST-BZIP40诱饵蛋白。利用GST-CsBZIP40诱饵蛋白从侵染溃疡病菌后柑橘总蛋白和未接菌柑橘总蛋白中成功钓取蛋白,并用LC-MS/MS检测。经过比对、注释和筛选,在柑橘溃疡病菌侵染过程中与GST-BZIP40特异结合的蛋白有53个,这些蛋白参与多个分子功能和通路。在这53个蛋白中,有6个蛋白(Cs1g02310、Cs3g05280、Cs3g23950、Cs6g13880、Cs7g12130、orange1.1t04973)可能与植物抗病性密切相关。数据库中已经证明53个蛋白中44个与CsBZIP40有直接或间接的互作关系。【结论】溃疡病菌侵染过程中有53个蛋白与CsBZIP40互作,根据注释6个蛋白与植物抗病性密切相关,这些蛋白可能在提高柑橘生物胁迫抗逆性方面发挥着重要的作用。
窦万福,祁静静,胡安华,陈善春,彭爱红,许兰珍,雷天刚,姚利晓,何永睿,李强. GST pull-down联合LC-MS/MS筛选柑橘抗溃疡病转录因子CsBZIP40的互作蛋白[J]. 中国农业科学, 2019, 52(13): 2243-2255.
DOU WanFu,QI JingJing,HU AnHua,CHEN ShanChun,PENG AiHong,XU LanZhen,LEI TianGang,YAO LiXiao,HE YongRui,LI Qiang. Screening of Interacting Proteins of Anti-Canker Transcription Factor CsBZIP40 in Citrus by GST Pull-Down Combined with LC-MS/MS[J]. Scientia Agricultura Sinica, 2019, 52(13): 2243-2255.
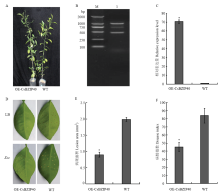
图1
过表达CsBZIP40柑橘植株验证和溃疡病抗性评价 A:过表达CsBZIP40转基因植株表型 Phenotypes of CsBZIP40 overexpression lines。B:转基因植株系中CsBZIP40的阳性检测The positive detection of CsBZIP40 in transgenic lines;M:Marker;1:阳性检测条带Positive detection band。C:过表达CsBZIP40的相对表达量 The relative expression of overexpressed CsBZIP40。D:接种溃疡病菌的转基因植株和野生型对照叶片 Disease spots of transgenic lines and the wild-type (WT) inoculated with LB and Xcc。E:接种溃疡病菌的转基因植株和野生型对照病斑面积 Lesion area of transgenic lines and the wild-type inoculated with Xcc;F:接种溃疡病菌的转基因植株和野生型对照病情指数 Disease index of transgenic lines and the wild-type inoculated with Xcc"

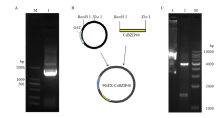
图2
融合表达载体构建。 A:CsBZIP40的PCR扩增 PCR amplification of CsBZIP40;M:2000 bp分子量标记2000 bp molecular marker;1:扩增产物,约1 530 bp Amplified product, about 1 530 bp。B:CsBZIP40-GST融合表达载体的构建 Construction of CsBZIP40-GST fusion expression vector。C:融合表达载体的酶切验证Enzymic digestion of CsBZIP40-GST vector;1:完整质粒条带The complete plasmid band;2:CsBZIP40-GST;M:10 000 bp分子量标记10 000 bp molecular marker"
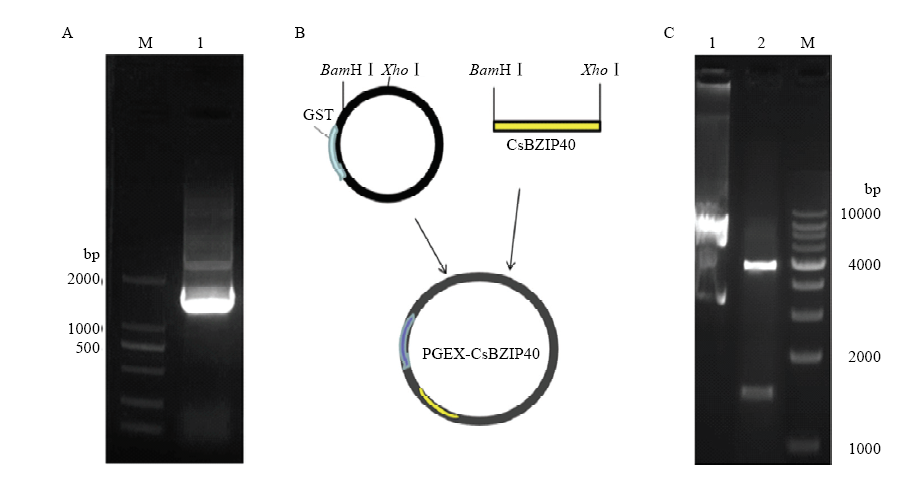
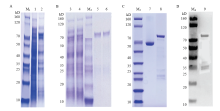
图3
融合蛋白诱导表达、纯化检测 A:GST-CsBZIP40融合蛋白表达 Expression of GST-CsBZIP40;M1:Marker;1:无IPTG诱导表达对照Expression of GST-CsBZIP40 without IPTG;2:15℃加IPTG诱导表达IPTG and 15℃ induced expression。B:诱饵蛋白纯化Bait protein purification;3:全菌破碎离心后上清Supernatant after centrifugation;4:上清同GST孵育后流出液Supernatant after incubation with GST;M2:Marker;5、6:40 HYPERLINK "https://www.baidu.com/s?wd=mm&tn=SE_PcZhidaonwhc_ngpagmjz&rsv_dl=gh_pc_zhidao" \t "_blank" mmol?L-1洗脱GST-CsBZIP40 40 HYPERLINK "https://www.baidu.com/s?wd=mm&tn=SE_PcZhidaonwhc_ngpagmjz&rsv_dl=gh_pc_zhidao" \t "_blank" mmol?L-1 eluent GST-CsBZIP40。C:诱饵蛋白纯化后浓度检测Concentration detection after purification of bait protein;M3:SDS-PAGE Marker;7:标准BSA Standard BSA;8:GST-CsBZIP40。D:诱饵蛋白纯化后纯度检测Fineness detection after purification of bait protein;M4:WB Marker;9:GST-CsBZIP40"
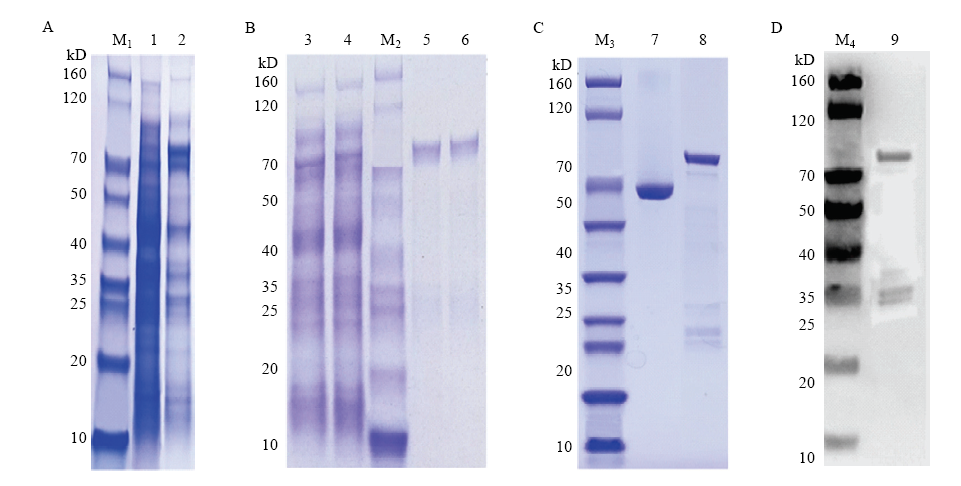
表1
抗病相关的CsBZIP40互作蛋白"
| 基因 ID Gene ID | 功能预测 Predicted protein function | 等电点 PI | 分子量 Molecular weight (kD) | 染色体位置 Chromosomal loci |
|---|---|---|---|---|
| Cs1g02310 | TGA1 resistance (SAR) via its interaction with NPR1 | 7.07 | 40.83 | chr1:1,637,432..1,643,794 |
| Cs3g05280 | Disease resistance protein | 5.83 | 130.03 | chr3:6,932,350..6,936,297 |
| Cs3g23950 | Transcription factor MYB | 8.75 | 28.06 | chr3:26,088,190..26,089,510 |
| Cs6g13880 | Peroxiredoxin-2 | 8.49 | 29.50 | chr6:15,280,950..15,284,180 |
| Cs7g12130 | Heat shock protein 83 | 5.34 | 90.12 | chr7:8,048,239..8,054,656 |
| orange1.1t04973 | Death-associated protein kinase 1 | 5.85 | 35.14 | chrUn:40,320,115..40,324,317 |
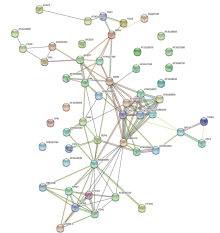
图7
CsBZIP40结合蛋白互作分析 节点代表溃疡病菌侵染过程中CsBZIP40的结合蛋白,圆圈中的图案为预测的蛋白三级结构,空白表示三级结构未知。不同颜色的连线表示不同数据来源 The circle in the figure is the binding protein of CsBZIP40. The pattern in the circle is the predicted protein tertiary structure, and the blank indicates that the tertiary structure is unknown. Line segments indicate that there is a clear link between the two proteins that has been proven or reported"

| [1] |
姚廷山, 周彦, 周常勇 . 柑橘溃疡病分化及其研究进展. 园艺学报, 2015,42(9):1699-1706.
doi: 10.16420/j.issn.0513-353x.2015-0128 |
|
YAO T S, ZHOU Y, ZHOU C Y . Research development of the differentiation and control of citrus bacterial canker disease. Acta Horticulturae Sinica, 2015,42(9):1699-1706. (in Chinese)
doi: 10.16420/j.issn.0513-353x.2015-0128 |
|
| [2] | BEHLAU F, CANTEROS B I, MINSAVAGE G V, JONES J B, GRAHAM J H . Molecular characterization of copper resistance genes from Xanthomonas citri subsp. citri and Xanthomonas alfalfae subsp. Citrumelonis. Applied and Environmental Microbiology, 2011,77(12):4089-4096. |
| [3] | 贾瑞瑞, 周鹏飞, 白晓晶, 陈善春, 许兰珍, 彭爱红, 雷天刚, 姚利晓, 陈敏, 何永睿, 李强 . 柑橘响应溃疡病菌转录因子CsBZIP40的克隆及功能分析. 中国农业科学, 2017,50(13):2488-2497. |
| JIA R R, ZHOU P F, BAI X J, CHEN S C, XU L Z, PENG A H, LEI T G, YAO L X, CHEN M, HE Y R, LI Q . Gene cloning and expression analysis of canker-related transcription factor CsBZIP40 in citrus. Scientia Agricultura Sinica, 2017,50(13):2488-2497. (in Chinese) | |
| [4] | 刘武, 戴良英 . 植物抗逆相关ERF转录因子研究综述. 中国农学通报, 2007,23(4):78-81. |
| LIU W, DAI L Y . ERF transcription factors related to plant stress-tolerance. Chinese Agricultural Science Bulletin, 2007,23(4):78-81. (in Chinese) | |
| [5] |
LEHTI-SHIU M, PANCHY N, WANG P P, UYGUN S, SHIU S H . Diversity, expansion, and evolutionary novelty of plant DNA-binding transcription factor families. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms, 2017,1860(1):3-20.
doi: 10.1016/j.bbagrm.2016.08.005 |
| [6] | MOLINA L, KAHMANN R . An Ustilago maydis gene involved in H2O2 detoxification is required for virulence. The Plant Cell, 2007,19(7):2293-2309. |
| [7] | LIN C H, YANG S L, CHUNG K R . The YAP1 homolog-mediated oxidative stress tolerance is crucial for pathogenicity of the necrotrophic fungus Alternaria alternata in citrus. Molecular Plant-Microbe Interactions, 2009,22(8):942-952. |
| [8] | LIAO Y, ZOU H F, WEI W, HAO Y J, TIAN A G, HUANG J, LIU Y F, ZHANG J S, CHEN S Y . Soybean GmbZIP44, GmbZIP62 and GmbZIP78 genes function as negative regulator of ABA signaling and confer salt and freezing tolerance in transgenic Arabidopsis. Planta, 2008,228(2):225-240. |
| [9] |
AMORIM L L B, DA FONSECA DOS SANTOS R, NETO J P B, GUIDA-SANTOS M, CROVELLA S, BENKO-ISEPPON A M . Transcription factors involved in plant resistance to pathogens. Current Protein and Peptide Science, 2017,18(4):335-351.
doi: 10.2174/1389203717666160619185308 |
| [10] | LI X L, FAN S H, HU W, LIU G Y, WEI Y X, HE C Z, SHI H T . Two cassava basic leucine zipper (bZIP) transcription factors (MebZIP3 and MebZIP5) confer disease resistance against cassava bacterial blight. Frontiers in Plant Science, 2017, 8: Article 2110. |
| [11] | FITZGERALD H A, CANLAS P E, CHERN M S, RONALD P C . Alteration of TGA factor activity in rice results in enhanced tolerance to Xanthomonas oryzae pv. oryzae. The Plant Journal, 2005,43:335-347. |
| [12] |
WANG H, ZENG X . Analysing protein-protein interactions using a GST-fusion protein to pull down the interacting target from the cell lysate. Technical Tips Online, 2000,5(1):26-30.
doi: 10.1016/S1366-2120(08)70153-3 |
| [13] | REN L, CHANG E, MAKKY K, HAAS A L, KABOORD B, QORONFLEH M W . Glutathione S-transferase pull-down assays using dehydrated immobilized glutathione resin. Analytical Biochemistry, 2003,322(2):164-169. |
| [14] | AKIYAMA T, PILLAI M A, SENTOKU N . Cloning, characterization and expression of OsGLN2, a rice endo-1,3-β-glucanase gene regulated developmentally in flowers and hormonally in germinating seeds. Planta, 2004,220(1):129-139. |
| [15] | 赵文, 王静, 李伟滔, 王静, 李晓冰, 朱立煌, 陈学伟 . 应用GST pull-down技术筛选水稻抗稻瘟病蛋白PID3互作蛋白的研究. 植物病理学报, 2015,45(5):476-485. |
| ZHAO W, WANG J, LI W T, WANG J, LI X B, ZHU L H, CHEN X W . Screening of rice proteins interacting with the rice blast resistance protein PID3 by GST pull-down. Acta Phytopathologica Sinica, 2015,45(5):476-485. (in Chinese) | |
| [16] |
林生, 陈婷, 周明明, 陈观水, 林文雄 . 果蔗SoSGT1与Gibberella fujikuroi侵染下果蔗叶片蛋白的互作研究. 热带亚热带植物学报, 2015,23(3):252-261.
doi: 10.11926/j.issn.1005-3395.2015.03.004 |
|
LIN S, CHEN T, ZHOU M M, CHEN G S, LIN W X . Studies on interaction between SoSGT1 and proteins in leaves of chewing cane infected by Gibberella fujikuroi. Journal of Tropical and Subtropical Botany, 2015,23(3):252-261. (in Chinese)
doi: 10.11926/j.issn.1005-3395.2015.03.004 |
|
| [17] | CHEN M, HE Y R, XU L Z, PENG A H, LEI T G, YAO L X, LI Q, ZHOU P F, BAI X J, DUAN M J, JIANG X Y, JIA R R, ZOU X P, CHEN S C . Cloning and expression analysis of citrus genes CsGH3.1 and CsGH3.6 responding to Xanthomonas axonopodis pv. citri infection. Horticultural Plant Journal, 2016,2(4):193-202. |
| [18] |
COX J, MANN M . MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nature Biotechnology, 2008,26(12):1367-1372.
doi: 10.1038/nbt.1511 |
| [19] | KANEHISA M, SATO Y, FURUMICHI M, MORISHIMA K, TANNBE M . New approach for understanding genome variations in KEGG. Nucleic Acids Research, 2018,47(Database issue):D590-D595. |
| [20] |
SZKLARCZYK D, MORRIS J H, COOK H, KUHN M, WYDER S, SIMONOVIC M, SANTOS A, DONCHEVA N T, ROTH A, BORK P, JENSEN L J, MERING C . The STRING database in 2017: quality- controlled protein-protein association networks, made broadly accessible. Nucleic Acids Research, 2017,45(Database issue):D362-D368.
doi: 10.1093/nar/gkw937 |
| [21] | XU Q, CHEN L L, RUAN X A, CHEN D J, ZHU A D, CHEN C L, BERTRAND D, JIAO W B, HAO B H, LYON M P, CHEN J J, GAO S, XING F, LAN H, CAHNG J W, GE X H, LEI Y, HU Q, MIAO YIN, WANG L, XIAO S X, BISWAS M K, ZENG W F, GUO F, CAO H B, YANG X M, XU X W, CEHNG Y J, ZHANG J, ROOSE M L, NAGARAJAN N, DENG X X, RUAN Y . The draft genome of sweet orange (Citrus sinensis). Nature Genetics, 2013,45(1):59-66. |
| [22] | LINDERMAYR C, SELL S, MULLER B, LEISTER D, DURNER J . Redox regulation of the NPR1-TGA1 system of Arabidopsis thaliana by nitric oxide. The Plant Cell, 2010,22(8):2894-2907. |
| [23] | SAFDAR W, MAJEED H, ALI B, HAVEED I . Molecular evolution and diversity of small heat shock proteins genes in plants. Pakistan Journal of Botany, 2012,44(Special issue):211-218. |
| [24] | RAFFAELE S, RIVAS S, ROBY D . An essential role for salicylic acid in AtMYB30-mediated control of the hypersensitive cell death program in Arabidopsis. FEBS Letters, 2006,580(14):3498-3504. |
| [25] |
NASEEM M, KALTDORF M, DANDEKAR T . The nexus between growth and defense signaling: auxin and cytokinin modulate plant immune response pathways. Journal of Experimental Botany, 2015,66(16):4885-4896.
doi: 10.1093/jxb/erv297 |
| [26] |
FEYS B J, PARKER J E . Interplay of signaling pathways in plant disease resistance. Trends in Genetics, 2000,16(10):449-455.
doi: 10.1016/S0168-9525(00)02107-7 |
| [27] |
DANGL J L, JONES J D . Plant pathogens and integrated defense responses to infection. Nature, 2001,411(6839):826-833.
doi: 10.1038/35081161 |
| [28] | MEUR G, BUDATHA M, CUPTA A D, PRAKASH S, KIRTI P B . Differential induction of NPR1 during defense responses in Brassica juncea. Physiological and Molecular Plant Pathology, 2006,68(4):128-137. |
| [29] |
MOU Z L, FAN W H, DONG X N . Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell, 2003,113(7):935-944.
doi: 10.1016/S0092-8674(03)00429-X |
| [30] | DESPRES C, DELONG C, GLAZE S, LIU E W, FOBERT P R . The Arabidopsis NPR1/NIM1 protein enhances the DNA binding activity of a subgroup of the TGA family of bZIP transcription factors. The Plant Cell, 2000,12(2):279-290. |
| [31] | JAKOBY M, WEISSHAAR B, DROGE-LASER W, VICENTE- CARBAJOSA J, TIEDEMANN J, KROJ T, PARCY F . BZIP transcription factors in Arabidopsis. TRENDS in Plant Science, 2002,7(3):106-111. |
| [32] | BARAH P, WINGE P, KUSNIERCZYK A, TRAN D H, BONES A M . Molecular signatures in Arabidopsis thaliana in response to insect attack and bacterial infection. PLoS ONE, 2013,8(3):e58987. |
| [33] | PERSAK H, PITZSCHKE A . Tight interconnection and multi-level control of Arabidopsis MYB44 in MAPK cascade signaling. PLoS ONE, 2013,8(2):e57547. |
| [34] |
NIJHAWAN A, JAIN M, TYAGI A K, KHURANA J P . Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice. Plant Physiology, 2008,146(2):333-350.
doi: 10.1104/pp.107.112821 |
| [35] | LIU J Y, CHEN N N, CHEN F, CAI B, SANTO S, TORNIELLI G B, PEZZOTTI M, CHENG Z M . Genome-wide analysis and expression profile of the bZIP transcription factor gene family in grapevine (Vitis vinifera). BMC Genomics, 2014,15:281. |
| [36] | CHEN X H, BARNABY J, SREEDHARAN A, HUANG X, ORBOVIC V, GROSSER J W, WANG N, DONG X, SONG W Y . Over-expression of the citrus gene CtNH1 confers resistance to bacterial canker disease. Physiological and Molecular Plant Pathology, 2013,84:115-122. |
| [37] | ORGEUR M, MARTENS M, LEONTE G, NASSARI S, BONNIN M A, BORNO S T, TIMMERMANN B, HECHT J, DUPREZ D, STRICKER S, . Genome-wide strategies identify downstream target genes of chick connective tissue-associated transcription factors. Development, 2018, 145 dev.161208. |
| [38] |
WOOD Z A, POOLE L B, KARPLUS P A . Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science, 2003,300(5619):650-653.
doi: 10.1126/science.1080405 |
| [39] |
DELLEDONNE M, XIA Y J, DIXON R A, LAMB C . Nitric oxide functions as a signal in plant disease resistance. Nature, 1998,394(6693):585-588.
doi: 10.1038/29087 |
| [40] |
AL-WHAIBI M H . Plant heat-shock proteins: A mini review. Journal of King Saud University—Science, 2011,23(2):139-150.
doi: 10.1016/j.jksus.2010.06.022 |
| [41] |
ZHANG A, ZHANG J, YE N H, CAO J, TAN M, ZAHNG J, JIANG M . ZmMPK5 is required for the NADPH oxidase-mediated self- propagation of apoplastic H2O2 in brassinosteroid-induced antioxidant defence in leaves of maize. Journal of Experimental Botany, 2010,61(15):4399-4411.
doi: 10.1093/jxb/erq243 |
| [1] | 李菲菲, 廉雪菲, 尹韬, 常媛媛, 金燕, 马小川, 陈岳文, 叶丽, 李云松, 卢晓鹏. 柑橘果实囊衣发育与化渣性的形成[J]. 中国农业科学, 2023, 56(2): 333-344. |
| [2] | 黄家权,李莉,吴丰年,郑正,邓晓玲. 携带不同原噬菌体的黄龙病菌在柑橘木虱体内的增殖及致病力[J]. 中国农业科学, 2022, 55(4): 719-728. |
| [3] | 蒋琪琪,许建建,苏越,张琦,曹鹏,宋晨虎,李中安,宋震. 柑橘黄化花叶病毒侵染性克隆构建及应用[J]. 中国农业科学, 2022, 55(24): 4840-4850. |
| [4] | 张琦,段玉,苏越,蒋琪琪,王春庆,宾羽,宋震. 基于柑橘叶斑驳病毒的表达载体构建及应用[J]. 中国农业科学, 2022, 55(22): 4398-4407. |
| [5] | 朱延松,张亚飞,程莉,杨胜男,赵婉彤,江东. 利用Target SSR-seq技术鉴定60份柑橘种质资源[J]. 中国农业科学, 2022, 55(22): 4458-4472. |
| [6] | 肖桂华,文康,韩健,郝晨星,叶蓉春,朱亦赤,萧顺元,邓子牛,马先锋. 钙对枳生长发育及柑橘溃疡病抗性的影响[J]. 中国农业科学, 2022, 55(19): 3767-3778. |
| [7] | 范子晗,罗雅尹,熊华烨,张育文,康福蓉,王昱桁,王洁,石孝均,张跃强. 酸性土壤硝化作用对柑橘铵毒害的效应[J]. 中国农业科学, 2022, 55(18): 3600-3612. |
| [8] | 杨程,龚桂芝,彭祝春,常珍珍,易璇,洪棋斌. 基于cpInDel标记和cpSSR标记的柑橘属及近缘属植物亲缘关系[J]. 中国农业科学, 2022, 55(16): 3210-3223. |
| [9] | 邹运乾,林子桢,许让伟,程运江. 替代柑橘聚乙烯薄膜单果套袋的涂膜剂研发及保鲜效果评价[J]. 中国农业科学, 2022, 55(12): 2398-2412. |
| [10] | 李镇希,李文婷,黄家权,郑正,许美容,邓晓玲. 膜吸附法结合可视化环介导等温扩增技术检测柑橘黄龙病菌[J]. 中国农业科学, 2022, 55(1): 74-84. |
| [11] | 段玉,许建建,马志敏,宾羽,周常勇,宋震. 柑橘叶斑驳病毒的逆转录重组酶聚合酶扩增检测[J]. 中国农业科学, 2021, 54(9): 1904-1912. |
| [12] | 赵珂,郑林,杜美霞,龙俊宏,何永睿,陈善春,邹修平. 柑橘SAR及其信号转导基因CsSABP2在黄龙病菌侵染中的响应特征[J]. 中国农业科学, 2021, 54(8): 1638-1652. |
| [13] | 胡冬梅,江东,李永平,彭磊,李冬云,朱延松,杨云光. 利用Target SSR-seq技术鉴定温州蜜柑芽变材料[J]. 中国农业科学, 2021, 54(23): 5083-5096. |
| [14] | 叶方婷,潘鑫峰,毛志君,李兆伟,范凯. 睡莲转录因子bZIP家族的分子进化以及功能分析[J]. 中国农业科学, 2021, 54(21): 4694-4708. |
| [15] | 张婧芸,刘语诺,王兆昊,彭爱红,陈善春,何永睿. 转CiNPR4基因柑橘抗溃疡病的机制解析[J]. 中国农业科学, 2021, 54(18): 3871-3880. |
|
||