













中国农业科学 ›› 2018, Vol. 51 ›› Issue (21): 4052-4064.doi: 10.3864/j.issn.0578-1752.2018.21.005
周茜茜1( ),邱化荣1,何晓文1,王宪璞1,刘秀霞1,李保华2,吴树敬1(
),邱化荣1,何晓文1,王宪璞1,刘秀霞1,李保华2,吴树敬1( ),陈学森1(
),陈学森1( )
)
收稿日期:2018-05-14
接受日期:2018-06-19
出版日期:2018-11-01
发布日期:2018-11-01
通讯作者:
周茜茜,吴树敬,陈学森
基金资助:
QianQian ZHOU1( ),HuaRong QIU1,XiaoWen HE1,XianPu WANG1,XiuXia LIU1,BaoHua LI2,ShuJing WU1(
),HuaRong QIU1,XiaoWen HE1,XianPu WANG1,XiuXia LIU1,BaoHua LI2,ShuJing WU1( ),XueSen CHEN1(
),XueSen CHEN1( )
)
Received:2018-05-14
Accepted:2018-06-19
Online:2018-11-01
Published:2018-11-01
Contact:
QianQian ZHOU,ShuJing WU,XueSen CHEN
摘要:
【目的】从‘富士’苹果中克隆MdWRKY40,研究其在水杨酸(SA)诱导条件下的表达模式及在苹果轮纹病抗病信号通路中的作用,为进一步揭示苹果的抗病机制提供理论依据。【方法】以‘富士’苹果为试材,克隆MdWRKY40的全长CDS序列,对其进行生物信息学分析,采用荧光定量PCR(qRT-PCR)分析其在苹果各组织中的表达水平,及对非生物胁迫SA的响应;研究外源SA处理对苹果叶片接种轮纹病菌(Botryosphaeria dothidea)的影响,并利用qRT-PCR检测病程相关蛋白基因的表达;将MdWRKY40在拟南芥中进行异源表达,对稳定表达的拟南芥幼苗叶片进行接菌处理,观察叶片发病程度及发病叶片数量,并采用qRT-PCR分析病程相关基因的表达;测量拟南芥幼苗的根系长度,并利用qRT-PCR检测生长素相关基因的表达。【结果】MdWRKY40包含长为858 bp完整的开放阅读框,编码286个氨基酸,预测其分子量为32.088 kD,等电点为8.15。系统进化树分析表明,MdWRKY40与白梨PbWRKY40序列相似性最高,亲缘关系最近,与拟南芥AtWRKY40在不同的分支上,亲缘关系较远,利用DANMAN软件进行MdWRKY40与AtWRKY40的多序列比对分析发现,MdWRKY40蛋白与AtWRKY40蛋白虽然都含有一个WRKYGQK保守结构域,但相似度仅为29.78%。qRT-PCR分析表明,MdWRKY40在根中的表达水平最高,在叶中的表达水平最低,并且在根、茎、叶中,SA均诱导了MdWRKY40的表达,且均呈现先升高后降低的趋势,在6 h时表达量最高;外源SA处理提高了苹果叶片对轮纹病菌的抗性,未处理的叶片发病率达92.59%,SA处理后发病率降至79.26%,并显著提高了病程相关蛋白基因MdPR2、MdPR5的表达量。与野生型相比,在拟南芥中异源过量表达MdWRKY40显著提高了拟南芥叶片对轮纹病菌的抗性,野生型拟南芥发病率达77.5%,而两个转基因拟南芥株系发病率仅为21.5%和17.4%,并显著提高了病程相关基因PR1、PR3、PR4的表达。过表达MdWRKY40的拟南芥植株根系生长受到抑制,培养7 d后转基因拟南芥主根长度分别是野生型拟南芥的39.9%和43.1%,培养10 d后主根长度分别是野生型拟南芥的58.5%和55.4%。基因表达结果显示,生长素合成相关基因AtTAA1和生长素运输相关基因AtPIN1、AtPIN2的表达水平在MdWRKY40过表达株系中显著低于野生型。【结论】MdWRKY40表达受SA和苹果轮纹病菌侵染诱导;MdWRKY40是苹果中重要的轮纹病抗病基因,该基因过表达显著提高对轮纹病菌的抗性;MdWRKY40具有调控植物根系生长发育的功能,可能通过下调生长素运输相关基因的表达影响植物根系生长发育。
周茜茜,邱化荣,何晓文,王宪璞,刘秀霞,李保华,吴树敬,陈学森. MdWRKY40介导提高苹果与拟南芥对轮纹病菌的免疫抗性[J]. 中国农业科学, 2018, 51(21): 4052-4064.
QianQian ZHOU,HuaRong QIU,XiaoWen HE,XianPu WANG,XiuXia LIU,BaoHua LI,ShuJing WU,XueSen CHEN. MdWRKY40 Mediated Improvement of the Immune Resistance of Apple and Arabidopsis thaliana to Botryosphaeria dothidea[J]. Scientia Agricultura Sinica, 2018, 51(21): 4052-4064.
表1
荧光定量PCR引物序列"
| 基因Gene | 正向引物序列 Forward primer sequence (5′-3′) | 反向引物序列Reverse primer sequence (5′-3′) | |
|---|---|---|---|
| MdWRKY40 | GAGAATGCAACCCTAAGATTCC | ATATGCATTTGGTGGTGGTG | |
| MdPR2 | ACTCACAGTCACCATCCTCAAC | AATCGAACATCAGCTGAATAGG | |
| MdPR5 | CTCACCTTGGCCATCCTCTT | TTGGATGCTAGTTCGAAGC | |
| AtPR1 | TCATGGCTAAGTTTGCTTCC | AATACACACGATTTAGCACC | |
| AtPR3 | ACGGAAGAGGACCAATGCAA | GAGCAGTCATCCAGAACCAAATC | |
| AtPR4 | ACCACCGCGGACTACTGTTC | ACCACCGCGGACTACTGTTC | |
| AtPIN1 | CGGTGGGAACAACATAAGCA | CACACTTGTTGGTGGCATCAC | |
| AtPIN2 | CCGTGGGGCTAAGCTTCTCATCT | AGCTTTCCGTCGTCTCCTATCTCC | |
| AtTAA1 | GATGAAGAATCGGTGGGAGAAGC | CGTCCCTAGCCACGCAAACGCAGG | |
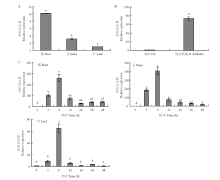
图3
MdWRKY40的组织表达分析及对水杨酸、轮纹病菌侵染诱导后的表达 柱上不同小写字母表示差异显著(P<0.05);**表示差异极显著(P<0.01)。下同 A:MdWRKY40的组织表达分析 Expression analysis of MdWRKY40 in different tissues;B:轮纹病菌处理‘嘎啦’叶片对MdWRKY40表达的影响 Effect of B. dothidea infection on the expression of MdWRKY40 in ‘Gala’ leaves;C:SA处理‘嘎啦’组培苗对MdWRKY40表达的影响Effect of SA on the expression of MdWRKY40 in ‘Gala’ tissue culture"
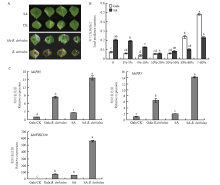
图4
SA处理苹果叶片接种轮纹病菌 A:SA处理苹果叶片接种轮纹病菌表型,SA代表仅用SA处理后的叶片,CK代表未做任何处理的叶片,SA-B. dothidea代表用SA处理后接种轮纹病菌的叶片,B. dothidea代表仅接种轮纹病菌的叶片SA treatment of apple leaves inoculated with B. dothidea phenotype, SA represents the leaves treated only with SA, CK represents the leaves without any treatment, SA-B. dothidea represents the leaves of inoculated with B. dothidea after treatment with SA, and B. dothidea represents the leaves of inoculated only with B. dothidea;B:SA处理苹果叶片接种轮纹病菌发病叶片统计,纵轴表示发病叶片占总叶片的比例,横轴表示病斑面积占叶片总面积的百分比SA treatment of apple leaves inoculated with B. dothidea leaf incidence statistics, the vertical axis indicates the proportion of diseased leaves to total leaves, and the horizontal axis indicates the percentage of lesion area to the total area of the leaves;C:qRT-PCR分析MdWRKY40和病程相关基因的表达水平 qRT-PCR analysis of MdWRKY40 and disease-associated gene expression levels"
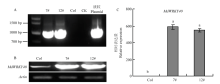
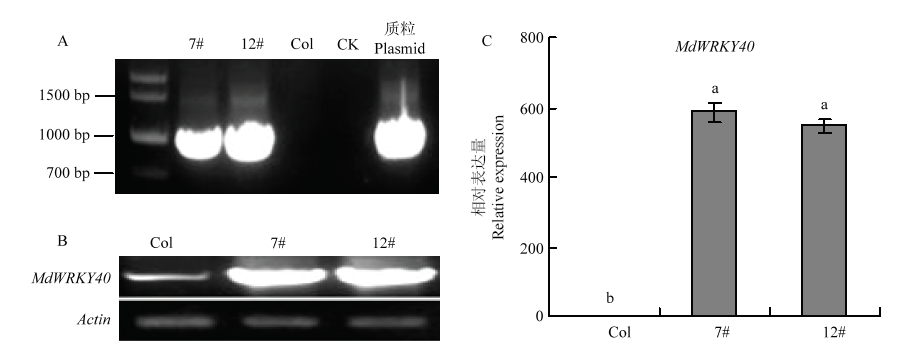
图5
转基因拟南芥的鉴定 A:PCR鉴定转基因拟南芥,7#、12#是两个转基因株系,Col代表野生型拟南芥,CK代表未加模板,质粒代表pCB302-35S-MdWRKY40-2HA Identification of transgenic A. thaliana by PCR, 7# and 12# are two transgenic lines, Col represents wild type A. thaliana, CK represents no template, and plasmid represents pCB302-35S-MdWRKY40-2HA;B:RT-PCR分析野生型拟南芥和异源表达株系中MdWRKY40的表达量 RT-PCR analysis of MdWRKY40 expression in wild type A. thaliana and heterologous expression lines;C:qRT-PCR分析野生型拟南芥和异源表达株系中MdWRKY40的表达量 qRT-PCR analysis of MdWRKY40 expression in wild type A. thaliana and heterologous expression lines"
| [1] | 国立耘, 李金云, 李保华, 张新忠, 周增强, 李广旭, 王英姿, 李晓军, 黄丽丽, 孙广宇, 文耀东 . 中国苹果枝干轮纹病发生和防治情况. 植物保护, 2009,35(4):120-123. |
| GUO L Y, LI J Y, LI B H, ZHANG X Z, ZHOU Z Q, LI G X, WANG Y Z, LI X J, HUANG L L, SUN G Y, WEN Y D . Investigations on the occurrence and chemical control of Botryosphaeria canker of apple in China. Plant Protection, 2009,35(4):120-123. (in Chinese) | |
| [2] | 张芮 . 苹果MdWRKY33基因在轮纹病抗性形成中的作用机制研究[D]. 泰安: 山东农业大学, 2015. |
| ZHANG R . The research on the mechanism of MdWRKY33 mediated disease resistance against the apple ring rot pathogenic fungi Botryosphaeria dothidea[D]. Taian: Shandong Agricultural University, 2015. ( in Chinese) | |
| [3] | TANG W, DING Z, ZHOU Z Q, WANG Y Z, GUO L Y . Phylogenetic and pathogenic analyses show that the causal agent of apple ring rot in China is Botryosphaeria dothidea. Plant Disease, 2012,96(4):486-496. |
| [4] | LI H Y, CAO R B, MU Y T . In vitro inhibition of Botryosphaeria dothidea and Lasiodiplodia theobromae, and chemical control of gummosis disease of Japanese apricot and peach trees in Zhejiang Province, China. Crop Protection, 1995,14(3):187-191. |
| [5] | LI Y, HAN L R, ZHANG Y, FU X, CHEN X, ZHANG L, MEI R, WANG Q . Biological control of apple ring rot on fruit by Bacillus amyloliquefaciens 9001. The Plant Pathology Journal, 2013,29(2):168-173. |
| [6] |
EULGEM T, SOMSSICH I E . Networks of WRKY transcription factors in defense signaling. Current Opinion of Plant Biology, 2007,10(4):366-371.
doi: 10.1016/j.pbi.2007.04.020 pmid: 17644023 |
| [7] |
薛华, 张红岩, 李小艳, 赵云, 王茂林 . 油菜矮秆突变WRKY转录因子cDNA克隆及表达分析. 西北植物学报, 2008,28(3):452-458.
doi: 10.3321/j.issn:1000-4025.2008.03.004 |
|
XUE H, ZHANG H Y, LI X Y, ZHAO Y, WANG M L . cDNA cloning and expression analysis of a dwarfism related WRKY transcription factor in Brassica napus L. Acta Botanica Boreali-Occidentalia Sinica, 2008,28(3):452-458. (in Chinese)
doi: 10.3321/j.issn:1000-4025.2008.03.004 |
|
| [8] | YU D, CHEN C, CHEN Z . Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. The Plant Cell, 2001,13(7):1527-1540. |
| [9] |
SHEN Q H, SAIJO Y, MAUCH S, BISKUP C, BIERI S, KELLER B, SEKI H, ÜLKER B, SOMSSICH I E, SCHULZE-LEFERT P . Nuclear activity of MLA immune receptors links isolate-specific and basal disease-resistance responses. Science, 2007,315(5815):1098-1103.
doi: 10.1126/science.1136372 pmid: 17185563 |
| [10] |
HAN M, KIM C Y, LEE J, LEE S K, JEON J S . OsWRKY42 represses OsMT1d and induces reactive oxygen species and leaf senescence in rice. Molecules and Cells, 2014,37(7):532-539.
doi: 10.14348/molcells.2014.0128 pmid: 4132305 |
| [11] |
DAI X, WANG Y, ZHANG W H . OsWRKY74, a WRKY transcription factor, modulates tolerance to phosphate starvation in rice. Journal of Experimental Botany, 2016,67(3):947-960.
doi: 10.1093/jxb/erv515 pmid: 4737085 |
| [12] |
KIM C Y, VO K T X, NGUYEN C D, JEONG D H, LEE S K, KUMAR M, KIM S R, PARK S H, KIM J K, JEON J SK . Functional analysis of a cold-responsive rice WRKY gene, OsWRKY71. Plant Biotechnology Reports, 2016,10(1):13-23.
doi: 10.1007/s11816-015-0383-2 |
| [13] |
RAINERI J, WANG S, PELEG Z, BLUMWALD E, CHAN R L . The rice transcription factor OsWRKY47 is a positive regulator of the response to water deficit stress. Plant Molecular Biology, 2015,88(4/5):401-413.
doi: 10.1007/s11103-015-0329-7 pmid: 25957211 |
| [14] | MIRABELLA R, RAUWERDA H, ALLMANN S, SCALA A, SPYROPOULOU E A, DE VRIES M, BOERSMA M R, BREIT T M, HARING M A, SCHUURINK R C . WRKY40 and WRKY6 act downstream of the green leaf volatile E-2-hexenal in Arabidopsis. The Plant Journal, 2015,83(6):1082-1096. |
| [15] |
ABBRUSCATO P, NEPUSZ T, MIZZI L, DEL CORVO M, MORANDINI P, FUMASONI I, MICHEL C, PACCANARO A, GUIDERDONI E, SCHAFFRATH U, MOREL J, PIFFANNELLI P, FAIVRE-RAMPANT O . OsWRKY22, a monocot WRKY gene, plays a role in the resistance response to blast. Molecular Plant Pathology, 2012,13(8):828-841.
doi: 10.1111/j.1364-3703.2012.00795.x pmid: 22443363 |
| [16] | CHOI C, HWANG S H, FANG I R, KWON S I, PARK S R, AHN I, KIM J B, HWANG D J . Molecular characterization of Oryza sativa WRKY6, which binds to W-box-like element 1 of the Oryza sativa pathogenesis-related (PR) 10a promoter and confers reduced susceptibility to pathogens. New Phytologist, 2015,208(3):846-859. |
| [17] | HAN M, RYU H S, KIM C Y, PARK D S, AHN Y K, JEON J S . OsWRKY30 is a transcription activator that enhances rice resistance to the Xanthomonas oryzae pathovar oryzae. Journal of Plant Biology, 2013,56(4):258-265. |
| [18] |
HWANG S H, KWON S I, JANG J Y, FANG I R, LEE H, CHOI C, PARK S R, AHN I, BAE S, HWANG D J . OsWRKY51, a rice transcription factor, functions as a positive regulator in defense response against Xanthomonas oryzae pv. oryzae. Plant Cell Reports, 2016,35(9):1975-1985.
doi: 10.1007/s00299-016-2012-0 pmid: 27300023 |
| [19] | LAN A, HUANG J, ZHAO W, PENG Y, CHEN Z, KANG D . A salicylic acid-induced rice (Oryza sativa L.) transcription factor OsWRKY77 is involved in disease resistance of Arabidopsis thaliana. Plant Biology, 2013,15(3):452-461. |
| [20] |
CIOLKOWSKI I, WANKE D, BIRKENBIHL R P, SOMSSICH I E . Studies on DNA-binding selectivity of WRKY transcription factors lend structural clues into WRKY-domain function. Plant Molecular Biology, 2008,68(1/2):81-92.
doi: 10.1007/s11103-008-9353-1 pmid: 2493524 |
| [21] |
RUSHTON P J, SOMSSICH I E, RINGLER P, SHEN Q J . WRKY transcription factors. Trends in Plant Science, 2010,15(5):247-258.
doi: 10.1016/j.tplants.2010.02.006 |
| [22] | 马丽娜, 张雄, 窦道龙, 柴春月 . 本氏烟NbWRKY40亚家族转录因子抗病相关功能研究. 植物病理学报, 2016,46(6):791-802. |
| MA L N, ZHANG X, DOU D L, CHAI C Y . Functional analysis of NbWRKY40 transcription factors of Nicotiana benthamiana. Acta Phytopathologica Sinica, 2016,46(6):791-802. (in Chinese) | |
| [23] |
XU X, CHEN C, FAN B, CHEN Z . Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. The Plant Cell, 2006,18(5):1310-1326.
doi: 10.1105/tpc.105.037523 pmid: 16603654 |
| [24] |
PANDEY S P, ROCCARO M, SCHöN M, LOGEMANN E, SOMSSICH I E . Transcriptional reprogramming regulated by WRKY18 and WRKY40 facilitates powdery mildew infection of Arabidopsis. The Plant Journal, 2010,64(6):912-923.
doi: 10.1111/j.1365-313X.2010.04387.x pmid: 21143673 |
| [25] | 罗昌国, 袁启凤, 裴晓红, 吴亚维, 郑伟, 章镇 . 富士苹果MdWRKY40b基因克隆及其对白粉病的抗性分析. 西北植物学报, 2013,33(12):2382-2387. |
| LUO C G, YUAN Q F, PEI X H, WU Y W, ZHENG W, ZHANG Z . Cloning of MdWRKY40b gene in Fuji apple and its response to powdery mildew stress. Acta Botanica Boreali-Occidentalia Sinica, 2013,33(12):2382-2387. (in Chinese) | |
| [26] | 刘威, 张骏, 顾冕, 徐国华 . 水稻转录因子WRKY-P1对地上部株型和根系构型的影响. 中国科技论文在线, 2016. |
| LIU W, ZHANG J, GU M, XU G H . The effect of a rice transcription factor WRKY-P1 on shoot and root architecture. Sciencepaper Online, 2016. ( in Chinese) | |
| [27] | 张高雷, 李保华, 董向丽, 王彩霞, 李桂舫, 国立耘 . 苹果轮纹病瘤组织形态研究. 植物病理学报, 2011,41(1):98-101. |
| ZHANG G L, LI B H, DONG X L, WANG C X, LI G F, GUO L Y . Microanatomy conformation of apple branch tumors caused by Botryosphaeria dothidea. Acta Phytopathologica Sinica, 2011,41(1):98-101. (in Chinese) | |
| [28] | ZHANG X, HENRIQUES R, LIN S S, NIU Q W, CHUA N H . Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nature Protocols, 2006,1(2):641-646. |
| [29] |
WU S, LU D, KABBAGE M, WEI H L, SWINGLE B, RECORDS A R, DICKMAN M, HE P, SHAN L . Bacterial effector HopF2 suppresses Arabidopsis innate immunity at the plasma membrane. Molecular Plant-Microbe Interactions, 2011,24(5):585-593.
doi: 10.1094/MPMI-07-10-0150 pmid: 21198360 |
| [30] | CAI H, YANG S, YAN Y, XIAO Z, CHENG J, WU J, QIU A, LAI Y, MOU S, GUAN D, HUANG R, HE S . CaWRKY6 transcriptionally activates CaWRKY40, regulates Ralstonia solanacearum resistance, and confers high-temperature and high-humidity tolerance in pepper. Journal of Experimental Botany, 2015,66(11):3163-3174. |
| [31] | WANG Y, DANG F, LIU Z, WANG X, EULGEM T, LAI Y, YU L, SHE J, SHI Y, LIN J, CHEN C, GUAN D, QIU A, HE S . CaWRKY58, encoding a group I WRKY transcription factor of Capsicum annuum, negatively regulates resistance to Ralstonia solanacearum infection. Molecular Plant Pathology, 2013,14(2):131-144. |
| [32] |
ZHENG Z Y, QAMAR S A, CHEN Z X, MENGISTE T . Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. The Plant Journal, 2006,48(4):592-605.
doi: 10.1111/j.1365-313X.2006.02901.x pmid: 17059405 |
| [33] | 石亚莉, 周会玲, 唐永萍, 贺军花, 马利菁 . 水杨酸诱导苹果采后灰霉病抗性研究. 西北农林科技大学学报 (自然科学版) , 2018,46(2):84-91, 103. |
| SHI Y L, ZHOU H L, TANG Y P, HE J H, MA L J . Induced resistance of postharvest apples to Botrytis cinerea induced by salicylic acid treatment. Journal of Northwest A&F University (Natural Science Edition), 2018,46(2):84-91, 103. (in Chinese) | |
| [34] |
KAZAN K . Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends in Plant Science, 2015,20(4):219-229.
doi: 10.1016/j.tplants.2015.02.001 pmid: 25731753 |
| [35] |
KHAN M I, FATMA M, PER T S, ANJUM N A, KHAN N A . Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Frontiers in Plant Science, 2015,6:462.
doi: 10.3389/fpls.2015.00462 pmid: 26175738 |
| [36] |
RAMAMOORTHY R, JIANG S Y, KUMAR N, VENKATESH P N, RAMACHANDRAN S . A comprehensive transcriptional profiling of the WRKY gene family in rice under various abiotic and phytohormone treatments. Plant and Cell Physiology, 2008,49(6):865-879.
doi: 10.1093/pcp/pcn061 pmid: 18413358 |
| [37] | 邱化荣, 周茜茜, 何晓文, 张宗营, 张世忠, 陈学森, 吴树敬 . 基于转录组分析苹果水杨酸特异响应基因MdWRKY40的启动子鉴定. 中国农业科学, 2017,50(20):3970-3990. |
| QIU H R, ZHOU Q Q, HE X W, ZHANG Z Y, ZHANG S Z, CHEN X S, WU S J . Identification of MdWRKY40 promoter specific response to salicylic acid by transcriptome sequencing. Scientia Agricultura Sinica, 2017,50(20):3970-3990. (in Chinese) | |
| [38] | ROBATZEK S, SOMSSICH I E . Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes and Development, 2002,16(9):1139-1149. |
| [39] |
GU Y, LI W, JIANG H, WANG Y, GAO H, LIU M, CHEN Q, LAI Y, HE C . Differential expression of a WRKY gene between wild and cultivated soybeans correlates to seed size. Journal of Experimental Botany, 2017,68(11):2717-2729.
doi: 10.1093/jxb/erx147 pmid: 28472462 |
| [40] |
ZHANG J, PENG Y, GUO Z . Constitutive expression of pathogen- inducible OsWRKY31 enhances disease resistance and affects root growth and auxin response in transgenic rice plants. Cell Research, 2008,18(4):508-521.
doi: 10.1038/cr.2007.104 pmid: 18071364 |
| [41] |
WEI L, WANG H, YU D . Arabidopsis WRKY transcription factors WRKY12 and WRKY13 oppositely regulate flowering under short- day conditions. Molecular Plant, 2016,9(11):1492-1503.
doi: 10.1016/j.molp.2016.08.003 pmid: 27592586 |
| [42] |
CHENG Y, AHAMMED G J, YU J, YAO Z, RUAN M, YE Q, LI Z, WANG R, FENG K, ZHOU G, YANG Y, DIAO W, WAN H . Putative WRKYs associated with regulation of fruit ripening revealed by detailed expression analysis of the WRKY gene family in pepper. Scientific Reports, 2016,6:39000.
doi: 10.1038/srep39000 pmid: 27991526 |
| [43] |
YANG Y, CHI Y, WANG Z, ZHOU Y, FAN B, CHEN Z . Functional analysis of structurally related soybean GmWRKY58 and GmWRKY76 in plant growth and development. Journal of Experimental Botany, 2016,67(15):4727-4742.
doi: 10.1093/jxb/erw252 pmid: 4973743 |
| [44] |
DEVAIAH B N, KARTHIKEYAN A S, RAGHOTHAMA K G . WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiology, 2007,143(4):1789-1801.
doi: 10.1104/pp.106.093971 pmid: 17322336 |
| [1] | 董永鑫,卫其巍,洪浩,黄莹,赵延晓,冯明峰,窦道龙,徐毅,陶小荣. 在中国大豆品种上创建ALSV诱导的基因沉默体系[J]. 中国农业科学, 2022, 55(9): 1710-1722. |
| [2] | 陈学森, 伊华林, 王楠, 张敏, 姜生辉, 徐娟, 毛志泉, 张宗营, 王志刚, 姜召涛, 徐月华, 李建明. 芽变选种推动世界苹果和柑橘产业优质高效发展案例解读[J]. 中国农业科学, 2022, 55(4): 755-768. |
| [3] | 路翔, 高源, 王昆, 孙思邈, 李连文, 李海飞, 李青山, 冯建荣, 王大江. 苹果栽培品种不同族系香气特征分析[J]. 中国农业科学, 2022, 55(3): 543-557. |
| [4] | 高小琴,聂继云,陈秋生,韩令喜,刘璐,程杨,刘明雨. 基于矿物元素指纹技术的‘富士’苹果产地溯源[J]. 中国农业科学, 2022, 55(21): 4252-4264. |
| [5] | 储宝华,曹富国,卞宁宁,钱谦,李中兴,李雪薇,刘泽远,马锋旺,管清美. 84个苹果栽培品种对斑点落叶病的抗性评价和全基因组关联分析[J]. 中国农业科学, 2022, 55(18): 3613-3628. |
| [6] | 胡亚丽,聂靖芝,吴霞,潘姣,曹珊,岳娇,罗登杰,王财金,李增强,张辉,吴启境,陈鹏. 水杨酸引发对红麻幼苗耐盐性的影响[J]. 中国农业科学, 2022, 55(14): 2696-2708. |
| [7] | 解斌,安秀红,陈艳辉,程存刚,康国栋,周江涛,赵德英,李壮,张艳珍,杨安. 不同苹果砧木对持续低磷的响应及适应性评价[J]. 中国农业科学, 2022, 55(13): 2598-2612. |
| [8] | 宋博文,杨龙,潘云飞,李海强,李浩,冯宏祖,陆宴辉. 农田景观格局对南疆苹果园梨小食心虫成虫种群动态的影响[J]. 中国农业科学, 2022, 55(1): 85-95. |
| [9] | 赵珂,郑林,杜美霞,龙俊宏,何永睿,陈善春,邹修平. 柑橘SAR及其信号转导基因CsSABP2在黄龙病菌侵染中的响应特征[J]. 中国农业科学, 2021, 54(8): 1638-1652. |
| [10] | 毕蒙蒙,刘迪,高歌,祝朋芳,毛洪玉. CmWRKY15-1通过水杨酸信号通路调控菊花白色锈病抗性[J]. 中国农业科学, 2021, 54(3): 619-628. |
| [11] | 沙仁和,兰黎明,王三红,罗昌国. 苹果转录因子MdWRKY40b抗白粉病的机理[J]. 中国农业科学, 2021, 54(24): 5220-5229. |
| [12] | 曹钰晗,李紫腾,张静怡,张静娜,胡同乐,王树桐,王亚南,曹克强. 我国苹果斑点落叶病菌携带dsRNA分析及一种dsRNA病毒的鉴定[J]. 中国农业科学, 2021, 54(22): 4787-4799. |
| [13] | 李紫腾,曹钰晗,李楠,孟祥龙,胡同乐,王树桐,王亚南,曹克强. 苹果锈果类病毒在7个品种苹果上的分子变异及系统发育关系[J]. 中国农业科学, 2021, 54(20): 4326-4336. |
| [14] | 张婧芸,刘语诺,王兆昊,彭爱红,陈善春,何永睿. 转CiNPR4基因柑橘抗溃疡病的机制解析[J]. 中国农业科学, 2021, 54(18): 3871-3880. |
| [15] | 宋春晖,陈晓菲,王枚阁,郑先波,宋尚伟,焦健,王苗苗,马锋旺,白团辉. 基于SLAF-seq技术鉴定苹果砧木耐涝候选基因[J]. 中国农业科学, 2021, 54(18): 3932-3944. |
|
||