













中国农业科学 ›› 2022, Vol. 55 ›› Issue (23): 4626-4639.doi: 10.3864/j.issn.0578-1752.2022.23.005
收稿日期:2022-06-28
接受日期:2022-08-08
出版日期:2022-12-01
发布日期:2022-12-06
联系方式:
张洁,E-mail:jiee@nwafu.edu.cn。
基金资助:
ZHANG Jie( ),JIANG ChangYue,WANG YueJin*(
),JIANG ChangYue,WANG YueJin*( )
)
Received:2022-06-28
Accepted:2022-08-08
Published:2022-12-01
Online:2022-12-06
摘要: 目的 欧洲葡萄作为世界葡萄主栽品种,具有产量高、品质佳的优点,但对病害抵抗能力差。白粉病是严重危害葡萄栽培的一种真菌性病害,中国野生葡萄资源丰富,可为抗病育种提供充足种质资源。论文旨在筛选调控抗白粉病的葡萄转录因子基因,探究转录因子基因调控抗白粉病的作用机理,为选育葡萄抗病品种提供优质的基因资源。方法 从中国野生毛葡萄‘商-24’中克隆得到转录因子基因VqWRKY6,使用DANMAN和MEGA-X软件对序列进行分析。利用PEG介导转化拟南芥原生质体进行亚细胞定位分析发挥转录调控作用的位置,利用酵母双杂交和双分子荧光互补试验证明VqWRKY6能够和转录因子VqbZIP1互作形成转录复合体。以感病葡萄‘赤霞珠’叶片为试材,通过农杆菌介导法瞬时转化到‘赤霞珠’葡萄叶片,叶片进行白粉菌(Uncinula necator)接种后,观察发病症状,用台盼蓝染色在显微镜下观察菌丝发育进程,使用DAB染色观察活性氧积累,比较共同过表达VqWRKY6和VqbZIP1的葡萄叶片、单独过表达VqWRKY6的葡萄叶片、单独过表达VqbZIP1的葡萄叶片和对照组叶片的差异。使用实时荧光定量PCR技术对抗病基因在白粉菌诱导下的表达水平进行分析。结果 VqWRKY6定位于葡萄2号染色体,编码342个氨基酸,属于WRKY家族的group Ⅲ亚家族。亚细胞定位和酵母转录激活试验证明,VqWRKY6在细胞核内发挥转录调控功能且在酵母中有转录激活活性。‘赤霞珠’叶片共同过表达VqWRKY6和VqbZIP1后,叶片表面白粉菌菌丝扩繁速率显著慢于单独过表达VqWRKY6和单独过表达VqbZIP1的叶片,共同过表达VqWRKY6和VqbZIP1的叶片组织中活性氧含量显著高于单独过表达VqWRKY6和单独过表达VqbZIP1的叶片;此外,VqWRKY6和VqbZIP1的协同调控能够激活茉莉酸(JA)途径的PR3、PR4,基因表达量显著上调。结论 VqWRKY6和VqbZIP1协同作用可能通过激活JA抗病途径,促进活性氧产生,增强抗病基因表达,抑制白粉菌的生长,从而提高葡萄对白粉病的抗性。因此,中国野生毛葡萄‘商-24’是重要的抗病种质资源,而VqWRKY6与VqbZIP1可作为重要的抗病基因资源。
张洁, 姜长岳, 王跃进. 中国野生毛葡萄转录因子VqWRKY6与VqbZIP1互作调控抗白粉病功能分析[J]. 中国农业科学, 2022, 55(23): 4626-4639.
ZHANG Jie, JIANG ChangYue, WANG YueJin. Functional Analysis of the Interaction Between Transcription Factors VqWRKY6 and VqbZIP1 in Regulating the Resistance to Powdery Mildew in Chinese Wild Vitis quinquangularis[J]. Scientia Agricultura Sinica, 2022, 55(23): 4626-4639.
表1
本研究所用引物"
| 引物名称 Primer name | 引物序列 Primer sequence (5′-3′) | 用途 Use |
|---|---|---|
| VqWRKY6-GFP-F-Kpn I | GGGGACGAGCTCGGTACCATGGACACTGGTTTGAAATGGCAG | pC2300 |
| VqWRKY6-GFP-R-Sal I | GCTCACCATGGTGTCGACGGAGAAAAATCCTGGGGTAT AAA | |
| VqbZIP1-GFP-F-Kpn I | GGGGACGAGCTCGGTACCTGGCGTCGTCGAAGGTGAT | pC2300 |
| VqbZIP1-GFP-R-Sal I | GCTCACCATGGTGTCGACCCACAGAACAGACTGCACT | |
| VqWRKY6-BD-F-EcoR I | CATATGGCCATGGAGGCGAATTCATGGACACTGGTTTGAAATGGCAG | pGBKT7 |
| VqWRKY6-BD-R-Sal I | ATGCGGCCGCTGCAGGTCGACGGAGAAAAATCCTGGGGTATTAAA | |
| VqbZIP1-AD-F-EcoR I | GCCATGGAGGCCAGTGAATTCATGGCGTCGTCGAAGGTGATG | pGADT7 |
| VqbZIP1-AD-R-BamH I | CTGCAGCTCGAGCTCGATGGATCCCCACAGAACAGACTGCACT | |
| VqbZIP1-pSPYNE-F-Kpn I | TCCGTCGACCTCGAGGGTACCATGGCGTCGTCGAAGGTGATG | pSPYNE |
| VqbZIP1-pSPYNE-R-Kpn I | CTCCTACCCGGGAGCGGTACCCCACAGAACAGACTGCACT | |
| VqWRKY6-pSPYCE-F-Xho I | GGGACTCTAGAGGATCTCGAGATGGACACTGGTTTGAAATGGCAG | pSPYCE |
| VqWRKY6-pSPYCE-R-Kpn I | ATCGTATGGGTACATGGTACCGGAGAAAAATCCTGGGGTATTAAA |
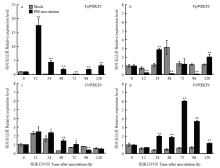
图1
不同葡萄叶片中WRKY6响应白粉菌诱导表达分析 a:商-24 Shang-24;b:白河-35-1 Baihe-35-1;c:赤霞珠Cabernet Sauvignon;d:无核白Thompson Seedless。Mock:无菌水处理葡萄叶片Grape leaves treated with sterile water;PM-inoculation:白粉菌接种后葡萄叶片Grape leaves inoculated with U. necator Expression analysis of WRKY6 in response to U. necator induction in different grape materials 显著性分析使用SPSS 26.0数据处理系统ANOVA单因素方差分析进行LSD检验Significance analysis was performed using One-Way ANOVA for LSD testing of SPSS 26.0 data processing system (* P<0.05; ** P<0.01)。图7同The same as Fig. 7"
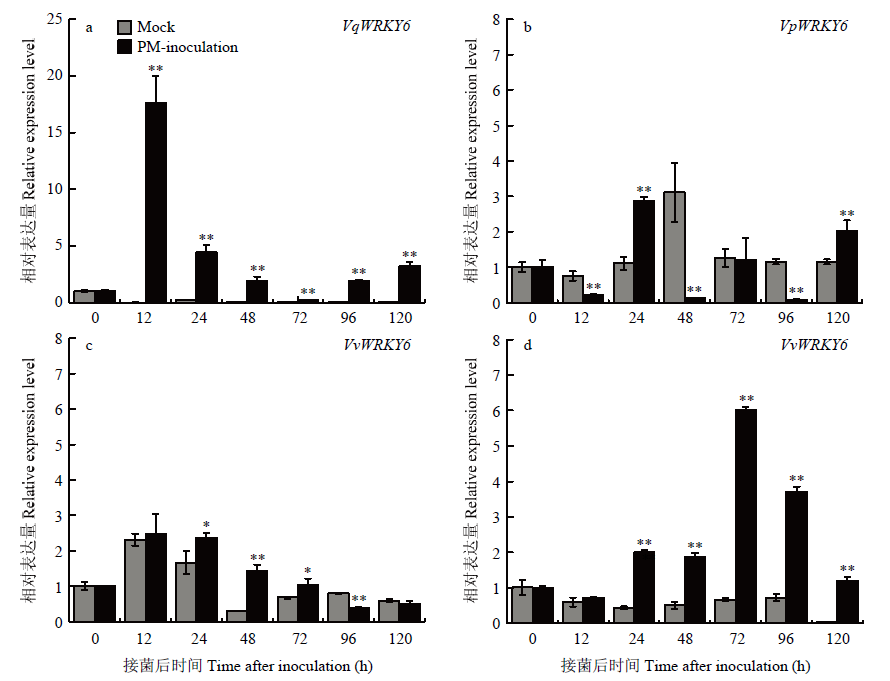
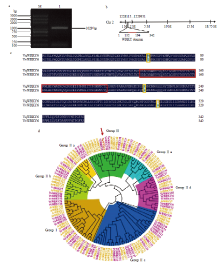
图2
中国野生毛葡萄VqWRKY6克隆及序列分析 a:M:DNA Marker;1:VqWRKY6克隆VqWRKY6 clone (1029 bp)。b:VqWRKY6定位于葡萄2号染色体,红色标注区域为WRKY结构域VqWRKY6 is located on the grape chromosome 2, and the red labeled area is the WRKY domain。c:中国野生毛葡萄VqWRKY6和欧洲葡萄VvWRKY6氨基酸序列对比,深蓝色部分表示序列一致,黄色方框标注处表示氨基酸序列突变位置,红色方框标注处为WRKY结构域Comparison of the amino acid sequences of Chinese wild V. quinquangularis VqWRKY6 and V. vinifera VvWRKY6, the dark blue part indicates the same sequence, the yellow box indicates the mutation position of the amino acid sequence, and the red box indicates the WRKY domain。d:中国野生毛葡萄转录因子VqWRKY6与欧洲葡萄和拟南芥WRKY家族成员聚类分析。VqWRKY6用红色箭头和红色字体标注,欧洲葡萄WRKY成员用紫色字体标注,拟南芥WRKY成员用黄色字体标注Cluster analysis of VqWRKY6 with WRKY family members from V. vinifera and A. thaliana. VqWRKY6 is marked with red arrow and red font, V. vinifera WRKY members are marked in purple font, and the WRKY members in A. thaliana are marked in yellow font"
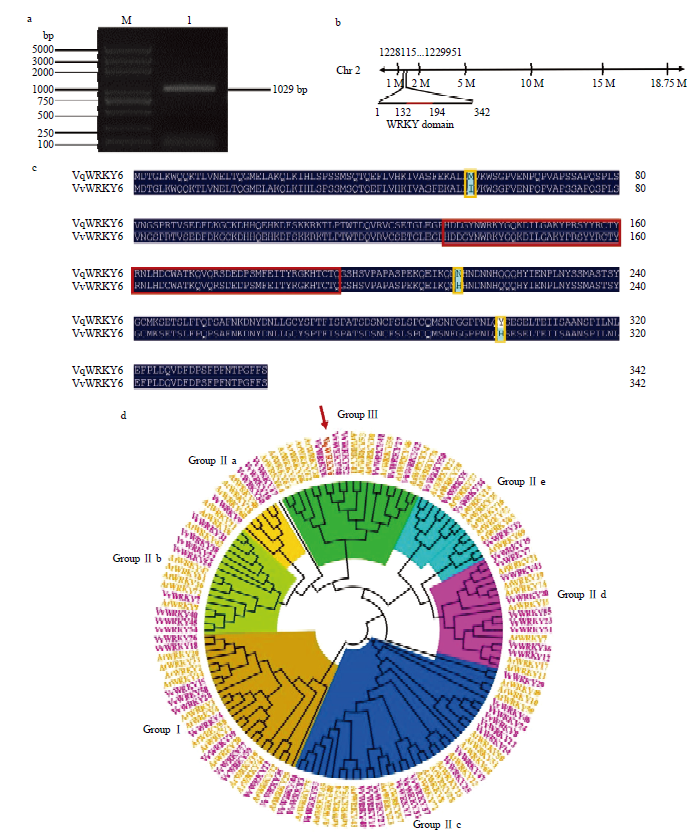
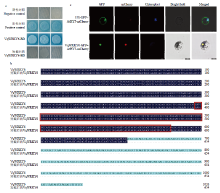
图3
中国野生毛葡萄转录因子VqWRKY6转录激活及亚细胞定位分析 a:VqWRKY6在酵母中转录激活功能分析,序列短截后的VqWRKY6转录自激活分析Function analysis of transcriptional activation on VqWRKY6 in yeast, and transcription activation analysis on VqWRKY6 after sequence short-cutting;b:短截后的VqWRKY6与全长VqWRKY6的序列比对分析Alignment of VqWRKY6 sequence in short-cutting and full-length;c:VqWRKY6在拟南芥原生质体中的亚细胞定位Subcellular localization of VqWRKY6 in A. thaliana protoplasts。GFP:绿色荧光信号Green fluorescence signal;mCherry:红色荧光信号Red fluorescence signal;Chloroplast:叶绿体自发荧光Chloroplast autofluorescence;Bright field:明场图Bright field plot;Merged:组合图Combined graph"
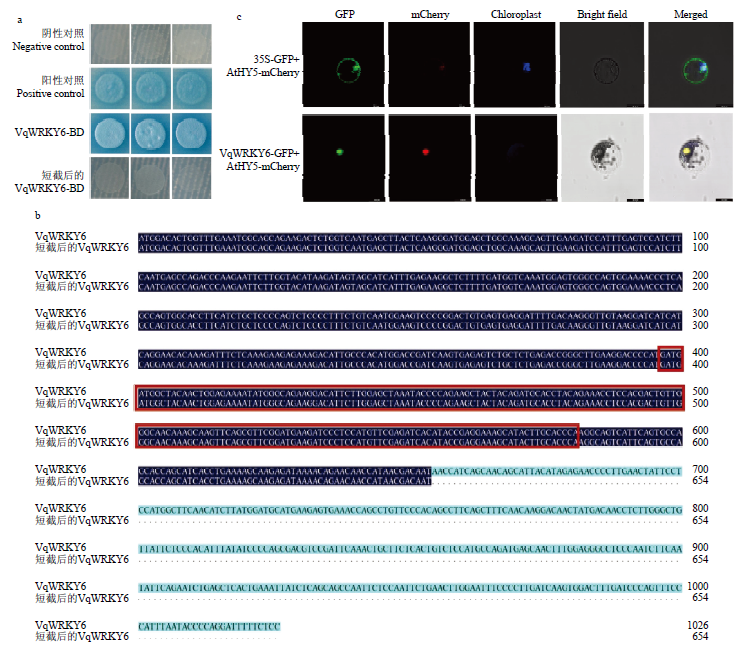
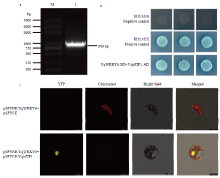
图5
双分子荧光互补技术验证中国野生毛葡萄中转录因子VqWRKY6与VqbZIP1蛋白互作 a:M:DNA Marker;1:VqbZIP1克隆VqbZIP1 clone (900 bp)。b:酵母双杂交验证VqWRKY6与VqbZIP1的互作The yeast two-hybrid was carried out to verify the interaction between VqWRKY6 and VqbZIP1。c:双分子荧光互补技术验证VqWRKY6与VqbZIP1的互作。pSPYNE-VqWRKY6和pSPYCE-VqbZIP1共转入拟南芥原生质体,以pSPYNE-VqWRKY6和pSPYCE共转入拟南芥原生质体作为对照To confirm the interaction between VqbZIP1 and VqWRKY6, the assay of bimolecular fluorescence complementation was performed. pSPYNE-VqWRKY6 and pSPYCE-VqbZIP1 were co-transferred to A. thaliana protoplasts, and pSPYNE-VqWRKY6 and pSPYCE were co-transferred to A. thaliana protoplasts as control"

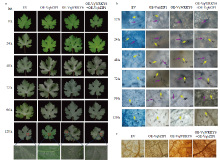
图6
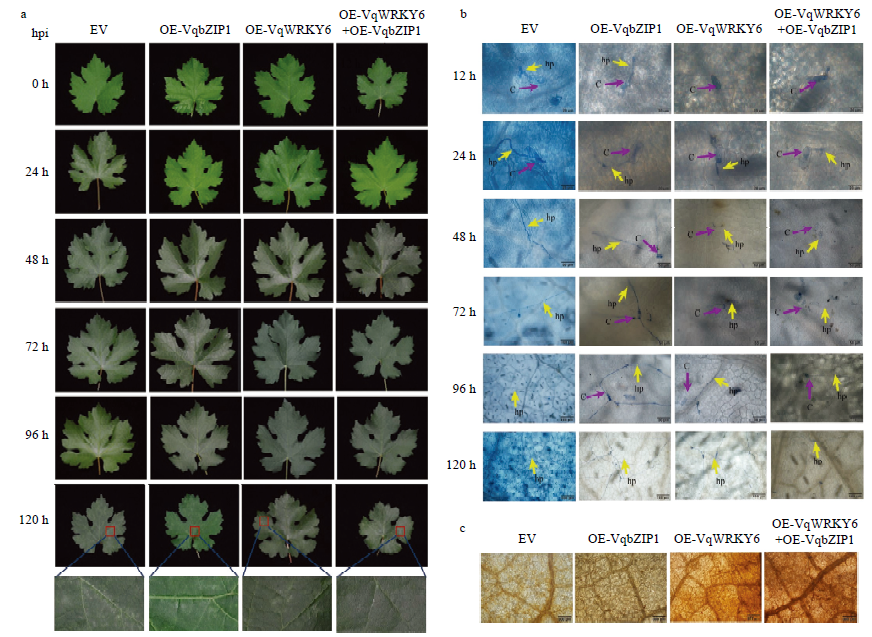
葡萄叶片中过表达VqWRKY6和VqbZIP1增强对白粉病的抗性 a:取瞬时转化后24 h的葡萄叶片进行人工接种白粉菌,对接种后0—120 h的叶片进行观察,红色方框标示白粉菌菌丝体Grape leaves were inoculated with U. necator at 24 h after transient transformation, the observation on leaves was performed at 0-120 h after inoculation; Red box indicates the mycelium of U. necator;EV:空载体对照Empty vector control;OE-VqbZIP1:单独过表达VqbZIP1 Overexpressing of VqbZIP1 alone;OE-VqWRKY6:单独过表达VqWRKY6 Overexpressing of VqWRKY6 alone;OE-VqWRKY6+OE-VqbZIP1:共同过表达VqWRKY6和VqbZIP1 Co-overexpressing of VqWRKY6 and VqbZIP1;hpi:白粉菌接种后时间Hours post inoculation。b:对瞬时转化后24 h的葡萄叶片进行人工接种白粉菌,显微观察菌丝发育生长进程Grape leaves were inoculated with U. necator at 24 h after transient transformation, and hyphae development and growth process were microscopically observed;c:分生孢子Conidium;hp:初级菌丝Primary hypha。c:DAB染色检测葡萄叶片组织中H2O2含量DAB staining is used to detect H2O2 content in grape tissues"
| [1] | 贺普超, 王跃进, 王国英, 任治邦, 和纯成. 中国葡萄属野生种抗病性的研究. 中国农业科学, 1991, 24(3): 50-56. |
| HE P C, WANG Y J, WANG G Y, REN Z B, HE C C. The studies on disease-resistance of Vitis wild species originated in China. Scientia Agricultura Sinica, 1991, 24(3): 50-56. (in Chinese) | |
| [2] |
ARMIJO G, SCHLECHTER R, AGURTO M, MUNOZ D, NUNEZ C, ARCE-JOHNSON P. Grapevine pathogenic microorganisms: Understanding infection strategies and host response scenarios. Frontiers in Plant Science, 2016, 7: 382.
doi: 10.3389/fpls.2016.00382 pmid: 27066032 |
| [3] |
DÉLYE C, LAIGRET F, CORIO-COSTET M F. A mutation in the 14 α-demethylase gene of Uncinula necator that correlates with resistance to a sterol biosynthesis inhibitor. Applied and Environmental Microbiology, 1997, 63(8): 2966-2970.
doi: 10.1128/aem.63.8.2966-2970.1997 |
| [4] |
DONALD T M, PELLERONE F, ADAM-BLONDON A F, BOUQUET A, THOMAS M R, DRY I B. Identification of resistance gene analogs linked to a powdery mildew resistance locus in grapevine. Theoretical and Applied Genetics, 2002, 104(4): 610-618.
pmid: 12582665 |
| [5] |
TAKSONYI P, KOCSIS L, MÁTYAS K K, TALLER J. The effect of quinone outside inhibitor fungicides on powdery mildew in a grape vineyard in Hungary. Scientia Horticulturae, 2013, 161: 233-238.
doi: 10.1016/j.scienta.2013.06.031 |
| [6] | WANG Y, LIU Y, P H. E, CHEN J, LAMIKANRA O, LU J. Evaluation of foliar resistance to Uncinula necator in Chinese wild Vitis species. Vitis, 1995, 34(3): 159-164. |
| [7] |
XU W, MA F, LI R, ZHOU Q, YAO W, JIAO Y, ZHANG C, ZHANG J, WANG X, XU Y, WANG Y. VpSTS29/STS 2 enhances fungal tolerance in grapevine through a positive feedback loop. Plant, Cell and Environment, 2019, 42(11): 2979-2998.
doi: 10.1111/pce.13600 |
| [8] |
EULGEM T. Regulation of the Arabidopsis defense transcriptome. Trends in Plant Science, 2005, 10(2): 71-78.
doi: 10.1016/j.tplants.2004.12.006 |
| [9] |
RYU H S, HAN M, LEE S K, CHO J I, RYOO N, HEU S, LEE Y H, BHOO S H, WANG G L, HAHN T R, JEON J S. A comprehensive expression analysis of the WRKY gene superfamily in rice plants during defense response. Plant Cell Reports, 2006, 25(8): 836-847.
doi: 10.1007/s00299-006-0138-1 |
| [10] |
EULGEM T, SOMSSICH I E. Networks of WRKY transcription factors in defense signaling. Current Opinion in Plant Biology, 2007, 10(4): 366-371.
pmid: 17644023 |
| [11] |
NAOUMKINA M A, HE X Z, DIXON R A. Elicitor-induced transcription factors for metabolic reprogramming of secondary metabolism in Medicago truncatula. BMC Plant Biology, 2008, 8: 132.
doi: 10.1186/1471-2229-8-132 |
| [12] |
RIECHMANN J L, RATCLIFFE O J. A genomic perspective on plant transcription factors. Current Opinion in Plant Biology, 2000, 3(5): 423-434.
pmid: 11019812 |
| [13] | 楚宗丽, 张睿男, 李亮杰, 孙君艳, 王付娟, 周强, 仝胜利. 小麦WRKY转录因子的鉴定及其在胚性愈伤组织形成中的表达分析. 麦类作物学报, 2021, 41(12): 1469-1478. |
| CHU Z L, ZHANG R N, LI L J, SUN J Y, WANG F J, ZHOU Q, TONG S L. Identification of wheat WRKY transcription factor and its expression analysis in embryonic callus formation. Journal of Triticeae Crops, 2021, 41(12): 1469-1478. (in Chinese) | |
| [14] |
SRIVASTAVA R, KUMAR S, KOBAYASHI Y, KUSUNOKI K, TRIPATHI P, KOBAYASHI Y, KOYAMA H, SAHOO L. Comparative genome-wide analysis of WRKY transcription factors in two Asian legume crops: Adzuki bean and mung bean. Scientific Reports, 2018, 8(1): 16971.
doi: 10.1038/s41598-018-34920-8 pmid: 30451872 |
| [15] |
GOFF S A, RICKE D O, LAN T H, PRESTING G G, WANG R, DUNN M, GLAZEBROOK J, SESSIONS A, OELLER P, VARMA H, et al.A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science, 2002, 296(5565): 92-100.
doi: 10.1126/science.1068275 |
| [16] |
GUO C L, GUO R R, XU X Z, GAO M, LI X Q, SONG J Y, ZHENG Y, WANG X P. Evolution and expression analysis of the grape (Vitis vinifera L.) WRKY gene family. Journal of Experimental Botany, 2014, 65(6): 1513-1528.
doi: 10.1093/jxb/eru007 |
| [17] |
CHUJO T, MIYAMOTO K, OGAWA S, MASUDA Y, SHIMIZU T, KISHI-KABOSHI M, TAKAHASHI A, NISHIZAWA Y, MINAMI E, NOJIRI H, YAMANE H, OKADA K. Overexpression of phosphomimic mutated OsWRKY53 leads to enhanced blast resistance in rice. PLoS ONE, 2014, 9(6): e98737.
doi: 10.1371/journal.pone.0098737 |
| [18] |
WANG D, JIANG C Y, LIU W D, WANG Y J. The WRKY 53 transcription factor enhances stilbene synthesis and disease resistance by interacting with MYB14 and MYB15 in Chinese wild grape. Journal of Experimental Botany, 2020, 71(10): 3211-3226.
doi: 10.1093/jxb/eraa097 |
| [19] | 吴凤颖, 刘梦琦, 王跃进. 中国野生毛葡萄芪合酶基因抗白粉病功能分析. 园艺学报, 2020, 47(2): 205-219. |
| WU F Y, LIU M Q, WANG Y J. Functional analysis of the stilbene synthase genes VqSTS12 and VqSTS25of the resistance to powdery mildew in Vitis quinquangularis. Acta Horticulture Sinica, 2020, 47(2): 205-219. (in Chinese) | |
| [20] |
HU Y R, DONG Q Y, YU D Q. Arabidopsis WRKY46 coordinates with WRKY70 and WRKY53 in basal resistance against pathogen Pseudomonas syringae. Plant Science, 2012, 185/186: 288-297.
doi: 10.1016/j.plantsci.2011.12.003 |
| [21] |
BIRKENBIHL R P, DIEZEL C, SOMSSICH I E. Arabidopsis WRKY33 is a key transcriptional regulator of hormonal and metabolic responses toward Botrytis cinerea infection. Plant Physiology, 2012, 159(1): 266-285.
doi: 10.1104/pp.111.192641 |
| [22] |
MENG Y, WISE R P. HvWRKY10, HvWRKY19, and HvWRKY28 regulate Mla-triggered immunity and basal defense to barely powdery mildew. Molecular Plant-Microbe Interactions, 2012, 25(11): 1492-1505.
doi: 10.1094/MPMI-04-12-0082-R |
| [23] |
BI M M, LI X Y, YAN X, LIU D, GAO G, ZHU P F, MAO H Y. Chrysanthemum WRKY15-1 promotes resistance to Puccinia horiana Henn. via the salicylic acid signaling pathway. Horticulture Research, 2021, 8: 6.
doi: 10.1038/s41438-020-00436-4 |
| [24] |
YIN W C, WANG X H, LIU H, WANG Y, NOCKER S, TU M X, FANG J H, GUO J Q, LI Z, WANG X P. Overexpression of VqWRKY31 enhances powdery mildew resistance in grapevine by promoting salicylic acid signaling and specific metabolite synthesis. Horticulture Research, 2022, 9: uhab064.
doi: 10.1093/hr/uhab064 |
| [25] |
CHRISTENSEN A B, CHO B H, NæSBY M, GREGERSEN P L, BRANDT J, MADRIZ-ORDEÑANA K, COLLINGE D B, THORDAL- CHRISTENSEN H. The molecular characterization of two barley proteins establishes the novel PR-17 family of pathogenesis-related proteins. Molecular Plant Pathology, 2002, 3(3): 135-144.
doi: 10.1046/j.1364-3703.2002.00105.x pmid: 20569319 |
| [26] |
VAN LOON L C, REP M, PIETERSE C M J. Significance of inducible defense-related proteins in infected plants. Annual Review of Phytopathology, 2006, 44: 135-162.
pmid: 16602946 |
| [27] |
HE M Y, XU Y, CAO J L, ZHU Z G, JIAO Y T, WANG Y J, GUAN X, YANG Y Z, XU W R, FU Z F. Subcellular localization and functional analyses of a PR10 protein gene from Vitis pseudoreticulata in response to Plasmopara viticola infection. Protoplasma, 2013, 250(1): 129-140.
doi: 10.1007/s00709-012-0384-8 |
| [28] | 马辉. 中国野生华东葡萄VpR82H基因的克隆与功能分析[D]. 杨凌: 西北农林科技大学, 2014. |
| MA H. Molecular cloning and functional analysis of VpR82H gene in Chinese wild Vitis pseudoreticulata[D]. Yangling: Northwest A&F University, 2014. (in Chinese) | |
| [29] | 刘兵, 李梦媛, 张娜, 尚博兴, 刘国甜, 徐炎. 中国野生葡萄抗霜霉病相关基因VpPR4b及其启动子的克隆和功能分析. 园艺学报, 2021, 48(2): 265-275. |
| LIU B, LI M Y, ZHANG N, SHANG B X, LIU G T, XU Y. Cloning and functional analysis of the CDS and promoter of VpPR4b gene response to downy mildew in Chinese wild grape. Acta Horticulture Sinica, 2021, 48(2): 265-275. (in Chinese) | |
| [30] |
MA F L, WANG L, WANG Y J. Ectopic expression of VpSTS29, a stilbene synthase gene from Vitis pseudoreticulata, indicates STS presence in cytosolic oil bodies. Planta, 2018, 248(1): 89-103.
doi: 10.1007/s00425-018-2883-0 |
| [31] | 姚文孔. 中国野生华东葡萄泛素连接酶基因VpPUB24功能研究[D]. 杨凌: 西北农林科技大学, 2017. |
| YAO W K. Function analyses of E3 ubiquitin ligase gene VpPUB24 from Chinese wild grape Vitis pseudoreticulata[D]. Yangling: Northwest A&F University, 2017. (in Chinese) | |
| [32] |
YOO S D, CHO Y H, SHEEN J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nature Protocols, 2007, 2(7): 1565-1572.
doi: 10.1038/nprot.2007.199 |
| [33] | 王跃进, 贺普超, 张剑侠. 葡萄抗白粉病鉴定方法的研究. 西北农林科技大学学报, 1999, 27(5): 6-10. |
| WANG Y J, HE P C, ZHANG J X. Studies on the methods of resistance to Uncinula necator in Vitis. Journal of Northwest A&F University, 1999, 27(5): 6-10. (in Chinese) | |
| [34] | MICALI C, GÖLLNER K, HUMPHRY M, CONSONNI C, PANSTRUGA R. The powdery mildew disease of Arabidopsis: A paradigm for the interaction between plants and biotrophic fungi//The Arabidopsis Book. The American Society of Plant Biologists, 2008, 6: e0115. |
| [35] |
LEVINE A, TENHAKEN R, DIXON R, LAMB C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell, 1994, 79(4): 583-593.
doi: 10.1016/0092-8674(94)90544-4 |
| [36] |
JIANG J J, MA S H, YE N H, JIANG M, CAO J S, ZHANG J H. WRKY transcription factors in plant responses to stresses. Journal of Integrative Plant Biology, 2017, 59(2): 86-101.
doi: 10.1111/jipb.12513 |
| [37] | SOMSSICH I E. Networks of transcriptional regulation underlying plant defense responses towards phytopathogens//GRASSER K D. Regulation of Transcription in Plants. Blackwell Publishing, 2006: 266-284. |
| [38] | 张远嬿. 苹果MdWRKY33基因的克隆与功能分析[D]. 沈阳: 沈阳农业大学, 2018. |
| ZHANG Y Y. Cloning and functional analysis of MdWRKY33 gene in apple[D]. Shenyang: Shenyang Agricultural University, 2018. (in Chinese) | |
| [39] | 周茜茜. 苹果轮纹病激发的SA特异性诱导表达基因MdWRKY40的抗病功能鉴定[D]. 泰安: 山东农业大学, 2019. |
| ZHOU Q Q. Identification of disease resistance of SA-specific inducible gene MdWRKY40 stimulated by Botryosphaeria dothidea[D]. Taian: Shandong Agricultural University, 2019. (in Chinese) | |
| [40] | 周茜茜, 邱化荣, 何晓文, 王宪璞, 刘秀霞, 李保华, 吴树敬, 陈学森. MdWRKY40介导提高苹果与拟南芥对轮纹病菌的免疫抗性. 中国农业科学, 2018, 51(21): 4052-4064. |
| ZHOU Q Q, QIU H R, HE X W, WANG X P, LIU X X, LI B H, WU S J, CHEN X S. MdWRKY40 mediated improvement of immune resistance of apple and Arabidopsis thaliana to Botryosphaeria dothidea. Scientia Agricultura Sinica, 2018, 51(21): 4052-4064. (in Chinese) | |
| [41] |
PANDEY S P, ROCCARO M, SCHON M, LOGEMANN E, SOMSSICH I E. Transcriptional reprogramming regulated by WRKY18 and WRKY40 facilitates powdery mildew infection of Arabidopsis. The Plant Journal, 2010, 64(6): 912-923.
doi: 10.1111/j.1365-313X.2010.04387.x |
| [42] |
LI H, XU Y, XIAO Y, ZHU Z G, XIE X Q, ZHAO H Q, WANG Y J. Expression and functional analysis of two genes encoding transcription factors, VpWRKY1 and VpWRKY2, isolated from Chinese wild Vitis pseudoreticulata. Planta, 2010, 232(6): 1325-1337.
doi: 10.1007/s00425-010-1258-y |
| [43] | 乔恒波. 中国野生毛葡萄转录因子WRKY3基因克隆与功能研究[D]. 杨凌: 西北农林科技大学, 2016. |
| QIAO H B. Cloning and function analysis of a WRKY3 transcription factor in Vitis quinquangularis[D]. Yangling: Northwest A&F University, 2016. (in Chinese) | |
| [44] |
DOU L L, GUO Y N, ONDATI E, PANG C Y, WEI H L, SONG M Z, FAN S L, YU S X. Identification and expression analysis of group Ⅲ WRKY transcription factors in cotton. Journal of Integrative Agriculture, 2016, 15(11): 2469-2480.
doi: 10.1016/S2095-3119(15)61306-5 |
| [45] | 魏娟娟, 杨伟, 潘宇, 张兴国, 李金华. 番茄WRKY41基因的克隆、表达分析与转基因植株的获得. 西南大学学报(自然科学版), 2017, 39(1): 46-54. |
| WEI J J, YANG W, PAN Y, ZHANG X G, LI J H. Cloning and expression analysis of a WRKY41 gene in tomato and its transfer into a tomato cultivar. Journal of Southwest University (Natural Science Edition), 2017, 39(1): 46-54. (in Chinese) | |
| [46] | WANG X, GUO R, TU M, WANG D, GUO C, WAN R, LI Z, WANG X. Ectopic expression of the wild grape WRKY transcription factor VqWRKY52 in Arabidopsis thaliana enhances resistance to the biotrophic pathogen powdery mildew but not to the necrotrophic pathogen Botrytis cinera. Frontiers in Plant Science, 2017, 8: 97. |
| [47] |
APEL K, HIRT H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology, 2004, 55: 373-399.
pmid: 15377225 |
| [48] |
MADER M, FUSSL R. Role of peroxidase in lignification of tobacco cells.Ⅱ. Regulation by phenolic compounds. Plant Physiology, 1982, 70(4): 1132-1134.
doi: 10.1104/pp.70.4.1132 |
| [49] |
FINATTO T, VIANA V E, WOYANN L G, BUSANELLO C, MAIA L C, OLIVEIRA A. Can WRKY transcription factors help plants to overcome environmental challenges? Genetics and Molecular Biology, 2018, 41(3): 533-544.
doi: S1415-47572018000400533 pmid: 30235398 |
| [50] |
LI J, BRADER G, PALVA E T. The WRKY 70 transcription factor: A node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. The Plant Cell, 2004, 16(2): 319-331.
doi: 10.1105/tpc.016980 |
| [51] |
LI J, BRADER G, KARIOLA T, PALVA E T. WRKY 70 modulates the selection of signaling pathways in plant defense. The Plant Journal, 2006, 46(3): 477-491.
doi: 10.1111/j.1365-313X.2006.02712.x |
| [52] |
MARCHIVE C, MZID R, DELUC L, BARRIEU F, PIRRELLO J, GAUTHIER A, CORIO-COSTET M F, REGAD F, CAILLETEAU B, HAMDI S, LAUVERGEAT V. Isolation and characterization of a Vitis vinifera transcription factor, VvWRKY1, and its effect on responses to fungal pathogens in transgenic tobacco plants. Journal of Experimental Botany, 2007, 58(8): 1999-2010.
doi: 10.1093/jxb/erm062 |
| [53] |
MØLLER S G, CHUA N H. Interactions and intersections of plant signaling pathways. Journal of Molecular Biology, 1999, 293(2): 219-234.
pmid: 10550205 |
| [54] |
CHEONG Y H, CHANG H S, GUPTA R, WANG X, ZHU T, LUAN S. Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiology, 2002, 129(2): 661-677.
doi: 10.1104/pp.002857 |
| [1] | 常春义, 曹元, Ghulam Mustafa, 刘红艳, 张羽, 汤亮, 刘兵, 朱艳, 姚霞, 曹卫星, 刘蕾蕾. 白粉病对小麦光合特性的影响及病害严重度的定量模拟[J]. 中国农业科学, 2023, 56(6): 1061-1073. |
| [2] | 葛天成, 尹飞, 胡琼波, 彭争科, 李振宇. MBF2转录调控小菜蛾谷胱甘肽S-转移酶代谢氯虫苯甲酰胺的功能[J]. 中国农业科学, 2023, 56(4): 665-673. |
| [3] | 冯向君, 王宏宇, 于静, 池春玉, 丁国华. 过表达拟南芥NPR1增强黄瓜对枯萎病和白粉病的抗性[J]. 中国农业科学, 2023, 56(14): 2701-2712. |
| [4] | 蔡苇荻,张羽,刘海燕,郑恒彪,程涛,田永超,朱艳,曹卫星,姚霞. 基于成像高光谱的小麦冠层白粉病早期监测方法[J]. 中国农业科学, 2022, 55(6): 1110-1126. |
| [5] | 冯子恒,宋莉,张少华,井宇航,段剑钊,贺利,尹飞,冯伟. 基于无人机多光谱和热红外影像信息融合的小麦白粉病监测[J]. 中国农业科学, 2022, 55(5): 890-906. |
| [6] | 康忱,赵雪芳,李亚栋,田哲娟,王鹏,吴志明. 黄瓜CC-NBS-LRR家族基因鉴定及在霜霉病和白粉病胁迫下的表达分析[J]. 中国农业科学, 2022, 55(19): 3751-3766. |
| [7] | 沙仁和,兰黎明,王三红,罗昌国. 苹果转录因子MdWRKY40b抗白粉病的机理[J]. 中国农业科学, 2021, 54(24): 5220-5229. |
| [8] | 王萍,郑晨飞,王娇,胡璋健,邵淑君,师恺. 番茄转录因子SlNAC29在调控植株衰老中的作用及机理[J]. 中国农业科学, 2021, 54(24): 5266-5276. |
| [9] | 丁茜,赵凯茜,王跃进. 中国野生毛葡萄芪合酶基因表达及对葡萄抗白粉病的影响[J]. 中国农业科学, 2021, 54(2): 310-323. |
| [10] | 孟祥坤,吴赵露,杨雪梅,官道杰,王建军. 二化螟P糖蛋白基因的克隆分析及对杀虫剂的诱导响应[J]. 中国农业科学, 2021, 54(19): 4121-4131. |
| [11] | 杜星,曾强,刘禄,李琦琦,杨柳,潘增祥,李齐发. 二花脸猪linc-NORFA核心启动子鉴定与转录调控分析[J]. 中国农业科学, 2021, 54(15): 3331-3342. |
| [12] | 王峰,王秀杰,赵胜男,闫家榕,卜鑫,张颖,刘玉凤,许涛,齐明芳,齐红岩,李天来. 光对园艺植物花青素生物合成的调控作用[J]. 中国农业科学, 2020, 53(23): 4904-4917. |
| [13] | 刘佼佼,王学敏,马琳,崔苗苗,曹晓宇,赵威. 紫花苜蓿MsWRKY42的分离、鉴定及其对非生物胁迫的响应[J]. 中国农业科学, 2020, 53(17): 3455-3466. |
| [14] | 亓飞,林姝,宋蒙飞,张孟茹,陈姝延,张乃心,陈劲枫,娄群峰. 黄瓜抗白粉病突变体筛选与鉴定[J]. 中国农业科学, 2020, 53(1): 172-182. |
| [15] | 冯婵婧,孙广正,王阳,马青. 番茄ShARPC5抗白粉病功能分析[J]. 中国农业科学, 2020, 53(1): 65-73. |
|
||