













中国农业科学 ›› 2025, Vol. 58 ›› Issue (24): 5175-5189.doi: 10.3864/j.issn.0578-1752.2025.24.006
王梦云( ), 邓力元, 余洋, 杨宇衡, 方安菲, 田斌年, 王静, 毕朝位*(
), 邓力元, 余洋, 杨宇衡, 方安菲, 田斌年, 王静, 毕朝位*( )
)
收稿日期:2025-08-19
接受日期:2025-09-26
出版日期:2025-12-22
发布日期:2025-12-22
通信作者:
联系方式:
王梦云,E-mail:wmengyun2002@163.com。
基金资助:
WANG MengYun( ), DENG LiYuan, YU Yang, YANG YuHeng, FANG AnFei, TIAN BinNian, WANG Jing, BI ChaoWei*(
), DENG LiYuan, YU Yang, YANG YuHeng, FANG AnFei, TIAN BinNian, WANG Jing, BI ChaoWei*( )
)
Received:2025-08-19
Accepted:2025-09-26
Published:2025-12-22
Online:2025-12-22
摘要:
【背景】稻瘟病由稻瘟病菌(Magnaporthe oryzae)引起,是严重威胁水稻产量的毁灭性真菌病害。先正达公司研发的苯并烯氟菌唑,属于全新结构的琥珀酸脱氢酶抑制剂(succinate dehydrogenase inhibitors,SDHIs)类杀菌剂,对稻瘟病菌抑制效果显著,但稻瘟病菌对其抗性机制研究比较匮乏。【目的】探究稻瘟病菌对苯并烯氟菌唑的抗性机制,为科学指导苯并烯氟菌唑在稻瘟病防治中的应用、延长其有效使用年限、保障防治效果提供理论依据。【方法】采用同源比对、分子对接和定点突变等方法,探究稻瘟病菌对不同SDHIs杀菌剂(苯并烯氟菌唑、联苯吡菌胺、氟唑菌酰胺、萎锈灵和氟吡菌酰胺)敏感性差异的原因,并分析不同位点氨基酸替换对苯并烯氟菌唑与稻瘟病菌亲和力的影响。【结果】75株稻瘟病菌对苯并烯氟菌唑高度敏感,平均EC50值为0.041 μg·mL-1,分布于0.018—0.068 μg·mL-1;5种SDHIs杀菌剂与MoSdh之间的相互作用存在差异,包括氢键、π-π堆积和疏水相互作用,二者亲和力由高到低依次为苯并烯氟菌唑、联苯吡菌胺、氟唑菌酰胺、萎锈灵和氟吡菌酰胺,与这5种SDHIs杀菌剂对稻瘟病菌的EC50值(从低到高)相对应。在稻瘟病菌琥珀酸脱氢酶复合体的8个位点中,共发现15种类型的氨基酸替换,其中,B亚基P198等7个位点氨基酸高度保守,C亚基S77位点存在物种差异。多数替换影响苯并烯氟菌唑与MoSdh的结合亲和力(主要与疏水相互作用及π-π堆积数量相关),其中MoSdhBP198Q、MoSdhBR243H等11种替换导致亲和力下降,SdhBH245D/L/Y突变菌株对苯并烯氟菌唑敏感性降低,与亲和力变化一致。【结论】分子对接可用于筛选防控稻瘟病的SDHIs杀菌剂;稻瘟病菌琥珀酸脱氢酶的B、C、D亚基发生多种氨基酸替换,包括MoSdhBP198Q、MoSdhBR243H、MoSdhBH245D/L/Y/Q、MoSdhBI247V/N、MoSdhCS77N和MoSdhDD122G/N,这些替换均可导致稻瘟病菌对苯并烯氟菌唑产生抗性。
王梦云, 邓力元, 余洋, 杨宇衡, 方安菲, 田斌年, 王静, 毕朝位. 稻瘟病菌琥珀酸脱氢酶不同位点氨基酸替换对苯并烯氟菌唑敏感性的影响[J]. 中国农业科学, 2025, 58(24): 5175-5189.
WANG MengYun, DENG LiYuan, YU Yang, YANG YuHeng, FANG AnFei, TIAN BinNian, WANG Jing, BI ChaoWei. Effects of Amino Acid Substitutions at Different Sites of Succinate Dehydrogenase on the Sensitivity of Magnaporthe oryzae to Benzovindiflupyr[J]. Scientia Agricultura Sinica, 2025, 58(24): 5175-5189.
表1
定点突变载体构建所用引物"
| 引物名称 Primer name | 序列 Sequence (5′-3′) | 产物 Production description |
|---|---|---|
| P1F | CGAGCTCCTCACATCTTGCCATCCTCGGTAC | 扩增构建pSKH载体的上游片段(1568 bp)Amplify the upstream fragment (1568 bp) for constructing the pSKH vector |
| P1R | TTGCGGCCGCTCATACGAAAGCCATCTCCTTCTTGAT | |
| P2F | CAAGCTTGGTGTGTGAGGAAGACGGTGATAGA | 扩增构建pSKH载体的下游片段(1128 bp)Amplify the downstream fragment (1128 bp) for constructing the pSKH vector |
| P2R | CCCCGGGACGCTTGTTGTTGTTAGGTTGTATTCG | |
| Jsac | TTAACCCTCACTAAAGGGAAC | 验证构建pSKH载体的上游片段(1649 bp)Verify the upstream fragment (1649 bp) of the constructed pSKH vector |
| Jnot | TTCAATATCATCTTCTGTCGAC | |
| Jsma | CCAGAATGCACAGGTACACTTGTT | 验证构建pSKH载体的下游片段(1276 bp)Verify the downstream fragment (1276 bp) of the constructed pSKH vector |
| Jhind | ACGACTCACTATAGGGCGAATTGG | |
| L1F | CATGAGCCTGTACCGTTGCCTCACCATTCTTAACTGCACAAG | 构建MoSdhBH245L质粒载体 Construct the MoSdhBH245L plasmid vector |
| L1R | CTTGTGCAGTTAAGAATGGTGAGGCAACGGTACAGGCTCATG | |
| Y1F | CCATGAGCCTGTACCGTTGCTACACCATTCTTAACTGCAC | 构建MoSdhBH245Y质粒载体 Construct the MoSdhBH245Y plasmid vector |
| Y1R | GTGCAGTTAAGAATGGTGTAGCAACGGTACAGGCTCATGG | |
| DF | TGATCCTCGGTGGCTACTG | 扩增原生质体转化所需的大片段(4597 bp) Amplify the large fragment (4597 bp) required for protoplast transformation |
| DR | CGCTTGTTGTTGTTAGGTTGTA | |
| JP1F | TCCACCATTGAATGCGACCATGAA | 验证转化子的上游片段(1859 bp) Verify the upstream fragment (1859 bp) of the transformant |
| JP1R | ATGTCCTCGTTCCTGTCTGCTAATAAG | |
| JP2F | CGGCGAAGCAGAAGAATAGC | 验证转化子的下游片段(2030 bp) Verify the downstream fragment (2030 bp) of the transformant |
| JP2R | CTGGAAGGGCGTGTTGAAC |
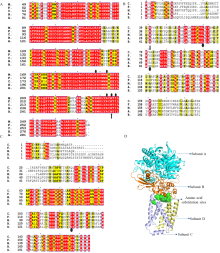
图2
不同作物病原真菌中琥珀酸脱氢酶复合体B、C、D亚基的同源比对 A:琥珀酸脱氢酶复合体B亚基的同源比对Homology alignment of succinate dehydrogenase complex B subunit;B:琥珀酸脱氢酶复合体C亚基的同源比对Homology alignment of succinate dehydrogenase complex C subunit;C:琥珀酸脱氢酶复合体D亚基的同源比对Homology alignment of succinate dehydrogenase complex D subunit;D:琥珀酸脱氢酶复合体中氨基酸替换位点的位置Position of amino acid substitution sites in succinate dehydrogenase complex。黑色圆点代表稻瘟病菌中氨基酸的替换位置,黑色箭头代表高粱炭疽病菌中氨基酸的替换位置Black dots represent the substitution position of amino acids in M. oryzae, black arrows represent the substitution position of amino acids in C. sublineola;A、B、C、F、M:分别代表链格孢、灰葡萄孢、高粱炭疽病菌、禾谷镰孢和稻瘟病菌A. alternata, B. cinerea, C. sublineola, F. graminearum and M. oryzae, respectively"

表2
野生型稻瘟病菌菌株对SDHIs的敏感性及SDHIs与其MoSdh的对接结合模式"
| 杀菌剂 Fungicide | 药剂结合腔 Binding pocket (≤5 Å) | 结合模式Binding mode | EC50 (μg·mL-1) | MoSdh与SDHIs的 结合亲和力 Docking affinity of MoSdh with SDHIs (kJ·mol-1) | ||||
|---|---|---|---|---|---|---|---|---|
| 疏水作用 Hydrophobic interaction | 氢键 Hydrogen bond | 其他作用力 Other interaction | ||||||
| 苯并烯氟菌唑 Benzovindiflupyr | B-W202, P198, I247 | B-P198, I247 | B-W202 | — | 0.027 | -30.209 | ||
| C-L66, V80 | C-L66, V80 | — | — | |||||
| D-Y123 | D-Y123 | D-Y123 | D-Y123 (π-π stacking) | |||||
| 联苯吡菌胺 Bixafen | C-L66, V80, R83 | C-L66, V80, R83 | — | — | 2.567 | -27.363 | ||
| D-Y123 | D-Y123 | D-Y123 | D-Y123 (π-π stacking) | |||||
| 氟唑菌酰胺 Fluxapyroxad | B-P198, I247 | B-P198, I247 | — | — | 8.230 | -24.727 | ||
| C-L66, L76, V80 | C-L66, L76, V80 | — | — | |||||
| D-Y123 | — | D-Y123 | D-Y123 (π-π stacking) | |||||
| 萎锈灵 Carboxin | B-H245, I247 | B-H245, I247 | — | — | 20.334 | -23.598 | ||
| C-R83, V80 | C-R83, V80 | — | C-R83 (π-π stacking) | |||||
| D-Y123 | — | D-Y123 | — | |||||
| 氟吡菌酰胺 Fluopyram | B-W202, S199, H245, I247 | B-H245, I247 | B-W202 | B-S199 (Halogen bond) | >200 | -23.221 | ||
| C-V80, R83 | C-V80 | — | C-R83 (π-π stacking) | |||||
| D-Y123 | — | — | D-Y123 (π-π stacking) | |||||
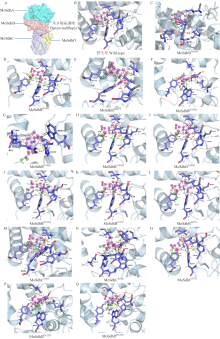
图4
苯并烯氟菌唑与不同类型稻瘟病菌MoSdh的分子对接结合模式 A:苯并烯氟菌唑与稻瘟病菌MoSdh对接的活性口袋位置Active pocket position of benzovindiflupyr docking with MoSdh of M. oryzae;B:苯并烯氟菌唑与野生型稻瘟病菌MoSdh的结合模式Binding mode of benzovindiflupyr to MoSdh of wild-type M. oryzae;C—Q:苯并烯氟菌唑与不同类型氨基酸替换菌株MoSdh的结合模式Binding mode of benzovindiflupyr to MoSdh of different types of amino acid substitution strains"

表3
苯并烯氟菌唑与不同类型稻瘟病菌MoSdh的分子对接"
| 突变方式 Mutation mode | 药剂结合腔 Binding pocket (≤5 Å) | 结合模式Binding mode | MoSdh与苯并烯氟菌唑的结合亲和力 Docking affinity of MoSdh with benzovindiflupyr (kJ·mol-1) | MoSdh与苯并烯氟菌唑的结合亲和力变化 Change of binding affinity between MoSdh and benzovindiflupyr (kJ·mol-1) | ||
|---|---|---|---|---|---|---|
| 疏水作用 Hydrophobic interaction | 氢键 Hydrogen bond | 其他作用力 Other interaction | ||||
| 野生型Wild-type | B-W202, P198, I247 | B-P198, I247 | B-W202 | D-Y123 (π-π stacking) | -30.209 | 0 |
| C-L66, V80 | C-L66, V80 | — | ||||
| D-Y123 | D-Y123 | D-Y123 | ||||
| SdhBH245D | B-W202, P198 | B-P198 | B-W202 | D-Y123 (π-π stacking) | -28.326 | 1.883 |
| C-L66, V80 | C-L66, V80 | — | ||||
| D-Y123 | — | D-Y123 | ||||
| SdhBH245L | B-W202, P198, I247 | B-P198, I247 | B-W202 | — | -29.539 | 0.670 |
| C-L66, V80 | C-L66, V80 | — | ||||
| D-Y123 | D-Y123 | D-Y123 | ||||
| SdhBH245Y | B-W202, P198 | B-P198 | B-W202 | — | -29.999 | 0.209 |
| C-L66, V80 | C-L66, V80 | — | ||||
| D-Y123 | D-Y123 | D-Y123 | ||||
| SdhBH245Q | B-W202, I247 | B-I247 | B-W202 | — | -29.748 | 0.460 |
| C-L66, V80 | C-L66, V80 | — | ||||
| D-Y123 | D-Y123 | D-Y123 | ||||
| SdhBP198Q | B-H245, W202 | B-H245 | B-W202 | C-R83 (π-π stacking) | -29.414 | 0.795 |
| C-Q82, R83 | C-Q82 | — | ||||
| SdhBN203H | B-W202, P198, I247 | B-P198, I247 | B-W202 | — | -30.376 | -0.167 |
| C-L66, V80 | C-L66, V80 | — | ||||
| D-Y123 | D-Y123 | D-Y123 | ||||
| SdhBN203K | B-W202, P198, I247 | B-P198, I247 | B-W202 | D-Y123 (π-π stacking) | -30.167 | 0.042 |
| C-L66, V80 | C-L66, V80 | — | ||||
| D-Y123 | D-Y123 | D-Y123 | ||||
| SdhBR243H | B-W202, P198, I247 | B-P198, I247 | B-W202 | D-Y123 (π-π stacking) | -27.949 | 2.259 |
| C-L66, V80 | C-L66, V80 | — | ||||
| D-Y123 | — | D-Y123 | ||||
| SdhBI247V | B-W202, P198, I247 | B-P198 | B-W202 | D-Y123 (π-π stacking) | -28.870 | 1.339 |
| C-L66, V80 | C-L66, V80 | — | ||||
| D-Y123 | D-Y123 | D-Y123 | ||||
| SdhBI247N | B-W202, P198 | B-P198 | B-W202 | — | -29.455 | 0.753 |
| C-L66, V80 | C-L66, V80 | N247 | ||||
| D-Y123 | D-Y123 | D-Y123 | ||||
| SdhCL66F | B-W202, P198, I247 | B-P198, W202, I247 | B-W202 | D-Y123 (π-π stacking) | -32.593 | -2.385 |
| C-F66, V80 | C-F66, V80 | — | ||||
| D-Y123 | D-Y123 | D-Y123 | ||||
| SdhCL66R | B-P198, W201, W202, I247 | B-P198, W201, I247 | B-W202 | D-D122 (Halogen bond) | -31.840 | -1.632 |
| C-R66, Y71, L76, V80 | C-R66, Y71, L76, V80 | C-R66 | ||||
| D-D122 | — | — | ||||
| SdhCS77N | B-W202, P198, I247 | B-P198, I247 | B-W202 | — | -29.957 | 0.251 |
| C-L66, V80 | C-L66, V80 | — | ||||
| D-Y123 | D-Y123 | D-Y123 | ||||
| SdhDD122G | B-W202, I247 | B-I247 | B-W202 | — | -27.656 | 2.552 |
| C-L66, V80 | C-L66, V80 | — | ||||
| D-Y123 | — | D-Y123 | ||||
| SdhDD122N | B-W202, P198, I247 | B-P198, I247 | B-W202 | — | -28.033 | 2.176 |
| C-L66, V80 | C-L66, V80 | — | ||||
| D-Y123 | — | D-Y123 | ||||
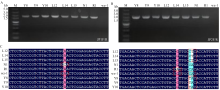
图5
氨基酸定点替换菌株的验证 A:SdhBH245L、SdhBH245Y、SdhBN203H和SdhBR243H氨基酸定点替换菌株上游验证电泳图Electrophoresis map of upstream validation of SdhBH245L, SdhBH245Y, SdhBN203H and SdhBR243H site-directed mutant strains;B:SdhBH245L、SdhBH245Y、SdhBN203H和SdhBR243H氨基酸定点替换菌株下游验证电泳图Electrophoresis map of downstream validation of SdhBH245L, SdhBH245Y, SdhBN203H and SdhBR243H site-directed mutant strains;C:SdhBH245L、SdhBH245Y、SdhBN203H和SdhBR243H氨基酸定点替换菌株测序图Sequencing of SdhBH245L, SdhBH245Y, SdhBN203H and SdhBR243H site-directed mutant strains。wz-1:野生型亲本菌株Wild-type parent strain;L12、L14、L15:SdhBH245L氨基酸定点替换菌株SdhBH245L site-directed mutant strain;Y8、Y9、Y10:SdhBH245Y氨基酸定点替换菌株SdhBH245Y site-directed mutant strain;N1:SdhBN203H氨基酸定点替换菌株SdhBN203H amino acid site-directed substitution strain;R1:SdhBR243H氨基酸定点替换菌株SdhBR243H amino acid site-directed substitution strain;CAC:组氨酸Histidine;CTC:亮氨酸Leucine;TAC:酪氨酸Tyrosine;AAC:天冬酰胺Asparagine;CGT:精氨酸Arginine;CAT:组氨酸Histidine"

表4
不同类型菌株对苯并烯氟菌唑的敏感性"
| 菌株 Strain | 基因型 Genotype | 回归方程 Equation of regression (y=) | R2 | EC50 (μg·mL-1) | RF | 抗性水平 Resistance level |
|---|---|---|---|---|---|---|
| wz-1 | — | 7.4741+1.5842x | 0.992 | 0.027 | — | — |
| H1 | — | 8.8909+2.5357x | 0.972 | 0.029 | 1 | S |
| D3 | SdhBH245D | 4.2376+0.9175x | 0.953 | 6.776 | 247 | HR |
| D5 | SdhBH245D | 4.3753+0.6525x | 0.947 | 9.066 | 331 | HR |
| Y8 | SdhBH245Y | 5.8840+1.3997x | 0.972 | 0.234 | 7 | LR |
| Y9 | SdhBH245Y | 5.8630+1.4149x | 0.973 | 0.246 | 9 | LR |
| Y10 | SdhBH245Y | 6.2961+1.6271x | 0.994 | 0.160 | 6 | LR |
| L12 | SdhBH245L | 3.5038+0.9303x | 0.999 | 40.579 | 1481 | VHR |
| L14 | SdhBH245L | 3.5434+0.9548x | 0.981 | 33.539 | 1224 | VHR |
| L15 | SdhBH245L | 3.3332+1.0327x | 0.989 | 41.117 | 1501 | VHR |
| N1 | SdhBN203H | 5.8713+1.4021x | 0.923 | 0.239 | 9 | LR |
| R1 | SdhBR243H | 7.8322+2.9574x | 0.917 | 0.110 | 4 | S |
| [1] |
|
| [2] |
|
| [3] |
褚晋, 闫晗, 徐晗, 缪建锟, 杨皓, 董海. 辽宁省稻瘟病菌对肟菌酯敏感性监测. 植物病理学报, 2021, 51(1): 95-103.
|
|
|
|
| [4] |
孙国昌, 杜新法, 陶荣祥, 孙漱沅. 水稻稻瘟病防治策略和21世纪研究展望. 植物病理学报, 1998, 28(4): 289-292.
|
|
|
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
朱凤, 田子华, 邰德良, 刘永锋. 从2014年稻瘟病重发谈今后防控对策的改进. 江苏农业科学, 2016, 44(8): 155-158.
|
|
|
|
| [10] |
张传清, 周明国, 朱国念. 稻瘟病化学防治药剂的历史沿革与研究现状. 农药学学报, 2009, 11(1): 72-80.
|
|
|
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance benzovindiflupyr. EFSA Journal, 2015, 13(3): 4043.
|
| [30] |
|
| [31] |
|
| [32] |
|
| [1] | 姚宏, 施寿荣, 赵茹茜. 基于网络药理学和分子对接探究绿原酸缓解鸡肠道炎症的作用机制[J]. 中国农业科学, 2025, 58(3): 600-616. |
| [2] | 刘卓琳, 刘红云. 基于网络药理学和分子对接探究芹菜素缓解奶牛热应激及低氧应激的潜力与机制[J]. 中国农业科学, 2024, 57(5): 1010-1022. |
| [3] | 徐重新, 金嘉凤, 孙晓明, 沈成, 张霄, 陈澄宇, 刘贤金, 刘媛. 基于Bt毒素的杀虫蛋白理性设计与创新应用策略[J]. 中国农业科学, 2024, 57(1): 96-125. |
| [4] | 刘瑞, 赵羽涵, 顾欣怡, 王艳霞, 靳学慧, 吴伟怀, 张亚玲. 黑龙江省和海南省稻瘟病菌中AVR-Pita家族的分布及变异分析[J]. 中国农业科学, 2023, 56(3): 466-480. |
| [5] | 李莉, 孙玲, 张金花, 邹晓威, 孙辉, 任金平, 姜兆远, 刘晓梅. 基于稻瘟病菌小种变化的吉林省主要粳稻品种抗性评价及利用价值分析[J]. 中国农业科学, 2023, 56(22): 4441-4452. |
| [6] | 刘瑞, 赵羽涵, 付忠举, 顾欣怡, 王艳霞, 靳学慧, 杨莹, 吴伟怀, 张亚玲. 黑龙江省和海南省PWL基因家族在稻瘟病菌中的分布及变异[J]. 中国农业科学, 2023, 56(2): 264-274. |
| [7] | 年和粉, 张钰析, 李伯辽, 陈秀琳, 罗坤, 李广伟. 李小食心虫GfunOBP2的原核表达及气味配体结合特性[J]. 中国农业科学, 2023, 56(12): 2302-2316. |
| [8] | 李桂香,李秀环,郝新昌,李智文,刘峰,刘西莉. 山东省多主棒孢对三种常用杀菌剂的敏感性监测及对氟吡菌酰胺的抗性[J]. 中国农业科学, 2022, 55(7): 1359-1370. |
| [9] | 汪文娟,苏菁,陈深,杨健源,陈凯玲,冯爱卿,汪聪颖,封金奇,陈炳,朱小源. 广东省侵染美香占2号的稻瘟病菌致病性及无毒基因变异分析[J]. 中国农业科学, 2022, 55(7): 1346-1358. |
| [10] | 吴云雨,肖宁,余玲,蔡跃,潘存红,李育红,张小祥,黄年生,季红娟,戴正元,李爱宏. 长江下游粳稻稻瘟病广谱抗性基因组合模式分析[J]. 中国农业科学, 2021, 54(9): 1881-1893. |
| [11] | 刘孝贺,仇贵生,佟兆国,张怀江,闫文涛,岳强,孙丽娜. 桃小食心虫化学感受蛋白CSP16配体结合特性[J]. 中国农业科学, 2021, 54(5): 945-958. |
| [12] | 秦健辉,李金桥,赵旭,李克斌,曹雅忠,尹姣. 铜绿丽金龟气味结合蛋白AcorOBP11的表达纯化及功能分析[J]. 中国农业科学, 2021, 54(14): 3017-3028. |
| [13] | 李祖任,罗丁峰,柏浩东,徐晶晶,韩进财,徐强,王若仲,柏连阳. 小飞蓬捕光叶绿素结合蛋白基因CcLhca-J9克隆及表达分析[J]. 中国农业科学, 2021, 54(1): 86-94. |
| [14] | 孟峰,张亚玲,靳学慧,张晓玉,姜军. 黑龙江省稻瘟病菌无毒基因AVR-Pib、AVR-Pik和AvrPiz-t的检测与分析[J]. 中国农业科学, 2019, 52(23): 4262-4273. |
| [15] | 孙炳学,石延霞,朱发娣,谢学文,柴阿丽,李宝聚. 多主棒孢SdhB-H278R突变位点AS-real-time PCR 定量检测体系的建立[J]. 中国农业科学, 2018, 51(24): 4647-4658. |
|
||