













中国农业科学 ›› 2020, Vol. 53 ›› Issue (24): 5125-5134.doi: 10.3864/j.issn.0578-1752.2020.24.015
• 畜牧·兽医·资源昆虫 • 上一篇
陈柳( ),倪征,余斌,华炯钢,叶伟成,云涛,刘可姝,朱寅初,张存(
),倪征,余斌,华炯钢,叶伟成,云涛,刘可姝,朱寅初,张存( )
)
收稿日期:2020-01-17
接受日期:2020-07-29
出版日期:2020-12-16
发布日期:2020-12-28
通讯作者:
张存
作者简介:陈柳,Tel:0571-86404257;E-mail: 基金资助:
CHEN Liu( ),NI Zheng,YU Bin,HUA JiongGang,YE WeiCheng,YUN Tao,LIU KeShu,ZHU YinChu,ZHANG Cun(
),NI Zheng,YU Bin,HUA JiongGang,YE WeiCheng,YUN Tao,LIU KeShu,ZHU YinChu,ZHANG Cun( )
)
Received:2020-01-17
Accepted:2020-07-29
Online:2020-12-16
Published:2020-12-28
Contact:
Cun ZHANG
摘要:
【背景】鸭瘟和鸭坦布苏病毒是鸭的两种重要传染病,鸭瘟属于疱疹病毒科,具有开发成病毒载体的优势。为了优化鸭坦布苏病毒E基因在重组鸭瘟病毒载体中的表达,之前探讨了不同形式鸭坦布苏病毒E蛋白在重组鸭瘟病毒载体中的表达,发现以鸭为宿主进行密码子优化的E基因C端截短形式(E451-dk,简称为Es)表达量最高。【目的】探讨不同启动子对Es 在重组鸭瘟病毒载体中表达的影响,为鸭瘟病毒—坦布苏病毒二联苗的研制奠定基础。【方法】将pCAG、 pSV40、pRSV、p1.8k(MDV)和pgB(MDV)启动子通过常规基因克隆的方法替换转移载体pEP-BGH-Es中的pCMV启动子,构建不同启动子调控Es表达的重组表达框pro-Es-BGH-pA。在鸭瘟病毒(DEV)疫苗株细菌人工染色体克隆pDEV-EF1的基础上,将5个重组表达框分别通过“Red E/T两步重组”克隆至pDEV-EF1突变体的US7和US8基因之间,构建了携带不同启动子调控的Es突变体克隆pDEV-pro-Es。用磷酸钙法转染鸡胚成纤维细胞(CEFs)拯救获得相应重组病毒rDEV-pro-Es,并对重组病毒感染细胞蚀斑大小和Es蛋白表达情况进行测定。【结果】将重组突变体克隆转染细胞拯救获得了5株重组病毒rDEV-pro-Es。Western blotting分析表明外源蛋白Es在 pRSV调控下表达量最高,其表达量较rDEV-Es提高了169.12%。【结论】完成了Es在重组鸭瘟病毒载体中高效表达启动子的筛选,获得了一种调控Es高效表达的启动子pRSV。同时也获得了一株高效表达鸭坦布苏病毒外源基因Es的重组鸭瘟病毒rDEV-pRSV-Es。
陈柳,倪征,余斌,华炯钢,叶伟成,云涛,刘可姝,朱寅初,张存. 重组鸭瘟病毒载体中筛选高效表达鸭坦布苏病毒E蛋白启动子[J]. 中国农业科学, 2020, 53(24): 5125-5134.
CHEN Liu,NI Zheng,YU Bin,HUA JiongGang,YE WeiCheng,YUN Tao,LIU KeShu,ZHU YinChu,ZHANG Cun. Optimized Promoter Regulating of Duck Tembusu Virus E Protein Expression Delivered by a Vectored Duck Enteritis Virus in vitro[J]. Scientia Agricultura Sinica, 2020, 53(24): 5125-5134.
表1
本文所用引物"
| 引物名称 Primer | 序列 Sequence | 引入位点 Sequence introduced |
|---|---|---|
| pCAG(MluI+) | 5′-cgACGCGTTAGTTATTAATAGTAATCAATTACG-3′ | Mlu I |
| PCAG(NheI-) | 5′-ctaGCTAGCGCCGCCGGTCACACGCCAGAAGCC-3′ | Nhe I |
| pRSV(BglII+) | 5′-gaAGATCTCTGCTCCCTGCTTGTGTGTTG-3′ | Bgl II |
| pRSV(NheI-) | 5′-ctaGCTAGCGTGCACACCAATGTGGTGAATG-3′ | Nhe I |
| pSV40(MluI+) | 5′-cgACGCGTCTGTGGAATGTGTGTCAGTTAGG-3′ | Mlu I |
| pSV40(NheI-) | 5′-ctaGCTAGCCGAAAATGGATATACAAGCTCCCGG-3′ | Nhe I |
| F(MDV p1.8k BglII+) | 5′-gaAGATCTTCGAGGCCACAAGAAATTAC-3′ | Bgl II |
| R(MDV p1.8k NheI-) | 5′-ctaGCTAGCGAGCATCGCGAAGAGAGAAG-3′ | Nhe I |
| F(MDV gB BglII+) | 5'-cgAGATCTCAAGTCTCACTCACAAATTTTTTC-3′ | Bgl II |
| R(MDV gB NheI-) | 5′-ctaGCTAGCAGTGAGATGATCTTAATGATGC-3′ | Nhe I |
| pDEV vac-in-s(p1.8k,pgB) | 5′-TACTAATTTAAGTGTGCAGCCTGGTTAACTGTATTATGCGCGGAGTGACGTCGACGGATCGGG-3′ | |
| pDEV vac-in-s | 5′-TACTAATTTAAGTGTGCAGCCTGGTTAACTGTATTATGCGCGGAGCGATGTACGGGCCAGATA-3′ | |
| pDEV vac-in-as | 5′- TCCGTAGTCTGGCCGGCAGTATGTTGGTGTTTAGTACTCCAAACCCA TAGAGCCCACCGCATCCCC-3′ | |
| JD-F | 5′-CTACCACAAGCGTCATCAACCA-3′ | |
| JD-R | 5′-TGTCCATTACCAAATCCGAAAA-3′ | |
| DEV-tk-F | 5′- GCTTCCCAGCAGCTCGTT-3′ | |
| DEV-tk-R | 5′- TCTCGTACTTCAGCGGCACA-3′ | |
| Dev UL44(BamHI+) | 5′-cgGGATCCATGGGGCCATTAGTGATGGTTG-3′ | BamH I |
| Dev UL44 (XhoI-) | 5′-ccgCTCGAGTCAAATAATATTGTCTGCTTTATC-3′ | XhoI |
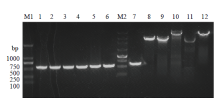
图2
重组BAC突变体克隆的PCR鉴定 泳道1—6 是以DEV-tk-F和DEV-tk-R引物对扩增片段;7—12 是以JD-F和JD-R引物对扩增片段。M1:DL2000(2000,1000, 750, 500, 250, 100 bp); 1. pDEV-EF1 (553 bp); 2. pDEV-pSV40. Es (553 bp); 3. pDEV- pRSV. Es (553 bp); 4. pDEV-pCAG. Es(553 bp); 5. pDEV-p1.8k (MDV). Es (553 bp); 6. pDEV-pgB(MDV). Es (553 bp); M2: 250 bp marker(4500, 3000, 2250, 1500, 1000, 750, 500, 250 bp); 7. pDEV-EF1(641 bp); 8. pDEV-pSV40. Es(2747 bp); 9. pDEV-pRSV. Es(2745 bp); 10. pDEV- pCAG. Es(3989 bp); 11. pDEV-p1.8k(MDV). Es (2687 bp);12. pDEV-pgB (MDV). Es (3037 bp)"
| [1] |
KALODIMOU G, VEIT S, JANY S, KALINKE U, BRODER C C, SUTTER G, VOLZ A. A soluble version of nipah virus glycoprotein G delivered by vaccinia virus MVA activates specific CD8 and CD4 T cells in mice. Viruses, 2020,12(1):26.
doi: 10.3390/v12010026 |
| [2] |
BERTRAN K, CRIADO M F, LEE D H, KILLMASTER L, Sà E SILVA M, LUCIO E, WIDENER J, PRITCHARD N, ATKINS E, MEBATSION T, SWAYNE D E. Protection of White Leghorn chickens by recombinant fowlpox vector vaccine with an updated H5 insert against Mexican H5N2 avian influenza viruses. Vaccine, 2020,38(6):1526-1534.
doi: 10.1016/j.vaccine.2019.11.072 pmid: 31862196 |
| [3] |
KIM J W, MORSHED R A, KANE J R, AUFFINGER B, QIAO J, LESNIAK M S. Viral vector production: adenovirus. Methods in Molecular Biology, 2016,1382:115-130.
doi: 10.1007/978-1-0716-0255-3_8 pmid: 32002905 |
| [4] |
LAROCCA R A, MENDES E A, ABBINK P, PETERSON R L, MARTINOT A J, IAMPIETRO M J, KANG Z H, AID M, KIRILOVA M, JACOB-DOLAN C, TOSTANOSKI L, BORDUCCHI E N, De La BARRERA R A, BAROUCH D H. Adenovirus vector- based vaccines confer maternal-fetal protection against Zika virus challenge in pregnant IFN-αβR-/- mice. Cell Host &Microbe, 2019,26(5):591-600.
doi: 10.1016/j.chom.2019.10.001 pmid: 31668877 |
| [5] | CHANG P, AMEEN F, SEALY J E, SADEYEN J R, BHAT S, LI Y, IQBAL M. Application of HDR-CRISPR/Cas9 and erythrocyte binding for rapid generation of recombinant turkey herpesvirus-vectored avian influenza virus vaccines. Vaccines (Basel), 2019,7(4):E192. |
| [6] |
ŚMIETANKA K, TYBOROWSKA J, OLSZEWSKA-TOMCZYK M, DOMAńSKA-BLICHARZ K, MINTA Z, RABALSKI L, CZARNOTA A, KUCHARCZYK K, SZEWCZYK B. A Recombinant turkey herpesvirus expressing F and HN genes of avian avulavirus-1 (AAvV-1) genotype VI confers cross-protection against challenge with virulent AAvV-1 genotypes IV and VII in chickens. Viruses, 2019,11(9):E784.
doi: 10.3390/v11090784 pmid: 31450681 |
| [7] |
KAMEL M, El-SAYED A. Utilization of herpesviridae as recombinant viral vectors in vaccine development against animal pathogens. Virus Research, 2019,270:197648.
doi: 10.1016/j.virusres.2019.197648 pmid: 31279828 |
| [8] |
LEMIALE F, ASEFA B, YE D, CHEN C, KOROKHOV N, HUMEAU L. An HIV-based lentiviral vector as HIV vaccine candidate: immunogenic characterization. Vaccine, 2010,28(8):1952-1961.
doi: 10.1016/j.vaccine.2009.10.089 |
| [9] |
LIN A, BALAZS A B. Adeno-associated virus gene delivery of broadly neutralizing antibodies as prevention and therapy against HIV-1. Retrovirology, 2018,15(1):66.
doi: 10.1186/s12977-018-0449-7 pmid: 30285769 |
| [10] | 展小过, 乔传玲, 杨焕良, 陈艳, 孔维, 辛晓光, 陈化兰. 表达H3N2亚型猪流感病毒HA基因重组腺病毒对小鼠免疫原性的研究. 中国农业科学, 2010,43(6):1235-1241. |
| ZHAN X G, QIAO C L, YANG H L, CHEN Y, KONG W, XIN X G, CHEN H L. Immunogenicity of a recombinant adenovirus expressing HA gene of H3N2 subtype swine influenza virus in mice. Scientia Agricultura Sinica, 2010,43(6):1235-1241. (in Chinese) | |
| [11] | 陈化兰, 马文军, 于康震. 表达禽流感病毒血凝素基因的重组禽痘病毒的构建, 中国农业科学, 2000,33(5):1-7. |
| CHEN H L, MA W J, YU K Z. Construction of a recombinant fowlpox virus expressing hemagglutinin gene of avian influenza virus. Scientia Agricultura Sinica, 2000,33(5):1-7. (in Chinese) | |
| [12] |
CHEN P, DING L, JIANG Y, ZENG X, DENG G, SHI J, LI Y, LIU L, ZHAO Y, HU Y, LIU J, CHEN H. Protective efficacy in farmed ducks of a duck enteritis virus-vectored vaccine against H5N1, H5N6, and H5N8 avian influenza viruses. Vaccine, 2019,37(40):5925-5929.
doi: 10.1016/j.vaccine.2019.08.026 pmid: 31471151 |
| [13] |
DING L, CHEN P, BAO X, LI A, JIANG Y, HU Y, GE J, ZHAO Y, WANG B, LIU J, CHEN H. Recombinant duck enteritis viruses expressing the Newcastle disease virus (NDV) F gene protects chickens from lethal NDV challenge. Veterinary Microbiology, 2019,232:146-150.
doi: 10.1016/j.vetmic.2019.04.022 pmid: 31030839 |
| [14] |
CHANG P, YAO Y, TANG N, SADEYEN J R, SEALY J, CLEMENTS A, BHAT S, MUNIR M, BRYABT J E, IQBAL M. the application of nhej-crispr/cas9 and cre-lox system in the generation of bivalent duck enteritis virus vaccine against avian influenza virus. Viruses, 2018,10(2):E81.
doi: 10.3390/v10020081 pmid: 29438322 |
| [15] |
ZOU Z, HUANG K, WEI Y, CHEN H, LIU Z, JIN M. Construction of a highly efficient CRISPR/Cas9-mediated duck enteritis virus- based vaccine against H5N1 avian influenza virus and duck Tembusu virus infection. Scientific Reports, 2017,7(1):1478.
doi: 10.1038/s41598-017-01554-1 pmid: 28469192 |
| [16] |
ZOU Z, MA J, HUANG K, CHEN H, LIU Z, JIN M. Live attenuated vaccine based on duck enteritis virus against duck hepatitis a virus types 1 and 3. Frontiers in Microbiology, 2016,7:1613.
doi: 10.3389/fmicb.2016.01613 pmid: 27777571 |
| [17] |
SUN Y, YANG C, LI J, LI L, CAO M, LI Q, LI H. Construction of a recombinant duck enteritis virus vaccine expressing hemagglutinin of H9N2 avian influenza virus and evaluation of its efficacy in ducks. Archives of Virology, 2017,162(1):171-179.
doi: 10.1007/s00705-016-3077-3 pmid: 27709401 |
| [18] |
LI H, WANG Y, HAN Z, WANG Y, LIANG S, JIANG L, HU Y, KONG X, LIU S. Recombinant duck enteritis viruses expressing major structural proteins of the infectious bronchitis virus provide protection against infectious bronchitis in chickens. Antiviral Research, 2016,130:19-26.
doi: 10.1016/j.antiviral.2016.03.003 pmid: 26946113 |
| [19] |
WANG J, GE A, XU M, WANG Z, QIAO Y, GU Y, LIU C, LIU Y, HOU J. Construction of a recombinant duck enteritis virus (DEV) expressing hemagglutinin of H5N1 avian influenza virus based on an infectious clone of DEV vaccine strain and evaluation of its efficacy in ducks and chickens. Virology Journal, 2015,12:126.
doi: 10.1186/s12985-015-0354-9 pmid: 26263920 |
| [20] |
ZOU Z, HU Y, LIU Z, ZHONG W, CAO H, CHEN H, JIN M. Efficient strategy for constructing duck enteritis virus-based live attenuated vaccine against homologous and heterologous H5N1 avian influenza virus and duck enteritis virus infection. Veterinary Research, 2015,46:42.
doi: 10.1186/s13567-015-0174-3 pmid: 25889564 |
| [21] |
WANG J, OSTERRIEDER N. Generation of an infectious clone of duck enteritis virus (DEV) and of a vectored DEV expressing hemagglutinin of H5N1 avian influenza virus. Virus Research, 2011,159(1):23-31.
doi: 10.1016/j.virusres.2011.04.013 |
| [22] |
LIU J, CHEN P, JIANG Y, WU L, ZENG X, TIAN G, GE J, KAWAOKA Y, BU Z, CHEN H. A duck enteritis virus-vectored bivalent live vaccine provides fast and complete protection against H5N1 avian influenza virus infection in ducks. Journal of Virology, 2011,85(21):10989-10998.
doi: 10.1128/JVI.05420-11 |
| [23] |
LIU X, WEI S, LIU Y, FU P, GAO M, MU X, LIU H, XING M, MA B, WANG J. Recombinant duck enteritis virus expressing the HA gene from goose H5 subtype avian influenza virus. Vaccine, 2013,31(50):5953-5959.
doi: 10.1016/j.vaccine.2013.10.035 pmid: 24144474 |
| [24] |
CHEN P, LIU J, JIANG Y, ZHAO Y, LI Q, WU L, HE X, CHEN H. The vaccine efficacy of recombinant duck enteritis virus expressing secreted E with or without PrM proteins of duck Tembusu virus. Vaccine, 2014,32(41):5271-5277.
doi: 10.1016/j.vaccine.2014.07.082 |
| [25] |
ZOU Z, LIU Z, JIN M. Efficient strategy to generate a vectored duck enteritis virus delivering envelope of duck Tembusu virus. Viruses, 2014,6(6):2428-2443.
doi: 10.3390/v6062428 pmid: 24956180 |
| [26] |
陈柳, 余斌, 倪征, 华炯钢, 叶伟成, 云涛, 张存. 表达小鹅瘟病毒VP2蛋白重组鸭瘟病毒的构建及其生物学特性. 中国农业科学, 2016,49(14):2813-2821.
doi: 10.3864/j.issn.0578-1752.2016.14.015 |
|
CHEN L, YUN B, NI Z, HUA J G, YE W C, YUN T, ZHANG C. Construction and characterization of a recombinant duck enteritis virus expressing VP2 gene of goose parvovirus. Scientia Agricultura Sinica, 2016, 49(14):2813-2821. (in Chinese)
doi: 10.3864/j.issn.0578-1752.2016.14.015 |
|
| [27] |
LI K, LIU Y, LIU C, GAO L, ZHANG Y, GAO Y, CUI H, QI X, ZHONG L, WANG X. Effects of different promoters on the protective efficacy of recombinant Marek's disease virus type 1 expressing the VP2 gene of infectious Bursal disease virus. Vaccine, 2016,34(47):5744-5750.
doi: 10.1016/j.vaccine.2016.10.008 pmid: 27742216 |
| [28] |
CHEN L, YU B, HUA J, et al. Construction of a full-length infectious bacterial artificial chromosome clone of duck enteritis virus vaccine strain. Virology Journal, 2013,10:328.
doi: 10.1186/1743-422X-10-328 pmid: 24195756 |
| [29] | 陈柳, 余斌, 倪征, 华炯钢, 叶伟成, 云涛, 张存. 表达鸭坦布苏病毒E 蛋白的重组鸭瘟病毒的构建及其生物学特性. 浙江农业学报, 2015,27(11):1889-1895. |
| CHEN L, YU B, NI Z, HUA J G, YE W C, YUN T, ZHANG C. Construction and characterization of a recombinant duck enteritis virus expressing E protein of duck Tembusu virus. Acta Agriculturae Zhejiangeensis, 2015,27(11):1889-1895.(in Chinese) | |
| [30] |
CHEN L, YU B, HUA J, NI Z, YE W, YUN T, ZHANG C. Optimized expression of duck Tembusu virus e gene delivered by a vectored duck enteritis virus in vitro. Molecular Biotechnology, 2019,61(10):783-790.
doi: 10.1007/s12033-019-00206-1 pmid: 31482466 |
| [31] | 马兴树. 禽传染病试验诊断技术. 北京: 化学工业出版社, 2006. |
| MA X S. Diagnostic Technique of Avian Infectious Disease. Beijing: Chemical Industry Press, 2006.(in Chinese) | |
| [32] | 余斌, 华炯钢, 刘跃生, 赵灵燕, 倪征, 叶伟成, 云涛, 陈柳, 徐辉, 张存. 鸭坦布苏病毒抗体间接ELISA 检测方法的建立和应用. 浙江畜牧兽医, 2014,5 : 1-6. |
| YU B, HUA J G, LIU Y S, ZHAO L Y, NI Z, YE W C, YUN T, CHEN L, XU H, ZHANG C. Establishment and application of indirect ELISA for detection of duck Tembusu virus antibody. Zhejiang Journal Animal Science and Veterinary Medicine, 2014,5:1-6. (in Chinese) | |
| [33] |
TISCHER B K, von EINEM J, KAUFER B, OSTERRIEDER N. Two-step red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. Biotechniques, 2006,40(2):191-197.
doi: 10.2144/000112096 pmid: 16526409 |
| [34] | TISCHER B K, SMITH G A, OSTERRIEDER N. En passant mutagenesis: a two step markerless red recombination system. Jeff Braman(ed.), In Vitro Mutagenesis Protocls: Third Edition, Methods in Molecular Biology, 2010,634:421-430. |
| [35] |
TSUKAMOTO K, SAITO S, SAEKI S, SATO T, TANIMURA N, ISOBE T, MASE M, IMADA T, YUASA N, YAMAGUCHI S. Complete, long-lasting protection against lethal infectious bursal disease virus challenge by a single vaccination with an avian herpesvirus vector expressing VP2 antigens. Journal of Virology, 2002,76(11):5637-5645.
doi: 10.1128/jvi.76.11.5637-5645.2002 pmid: 11991992 |
| [36] |
MA C, ZHANG Z, ZHAO P, DUAN L, ZHANG Y, ZHANG F, CHEN W, CUI Z. Comparative transcriptional activity of five promoters in BAC-cloned MDV for the expression of the hemagglutinin gene of H9N2 avian influenza virus. Journal of Virological Methods, 2014,206:119-127.
doi: 10.1016/j.jviromet.2014.05.023 |
| [37] |
LI L, ZHANG Y, DONG J, ZHANG J, ZHANG C, SUN M, CAO Y. The truncated E protein of DTMUV provide protection in young ducks. Veterinary Microbiology, 2020,240:108508.
doi: 10.1016/j.vetmic.2019.108508 pmid: 31902493 |
| [1] | 李旭飞,杨盛迪,李松琦,刘海楠,裴茂松,韦同路,郭大龙,余义和. 葡萄VlCKX4表达特性分析与转录调控预测[J]. 中国农业科学, 2023, 56(1): 144-155. |
| [2] | 闫强,薛冬,胡亚群,周琰琰,韦雅雯,袁星星,陈新. 大豆根特异性GmPR1-9启动子的鉴定及其在根腐病抗性中的应用[J]. 中国农业科学, 2022, 55(20): 3885-3896. |
| [3] | 徐献斌,耿晓月,李慧,孙丽娟,郑焕,陶建敏. 基于转录组分析ABA促进葡萄花青苷积累相关基因[J]. 中国农业科学, 2022, 55(1): 134-151. |
| [4] | 杜星,曾强,刘禄,李琦琦,杨柳,潘增祥,李齐发. 二花脸猪linc-NORFA核心启动子鉴定与转录调控分析[J]. 中国农业科学, 2021, 54(15): 3331-3342. |
| [5] | 卞书迅,韩晓蕾,袁高鹏,张利义,田义,张彩霞,丛佩华. 苹果U6启动子的克隆及功能分析[J]. 中国农业科学, 2019, 52(23): 4364-4373. |
| [6] | 杨志远,段会娟,王小蕾,刘立新,赵际成,潘洁,刘月焕,林健. 4株鸭坦布苏病毒的毒力、E基因序列和抗原差异性[J]. 中国农业科学, 2019, 52(23): 4406-4414. |
| [7] | 王小蕾, 刘月焕, 段会娟, 刘立新, 杨志远, 赵际成, 潘洁, 刘瑞华, 赵文奇, 田方杰, 吕金宝, 林健. 鸭坦布苏病毒的血凝性[J]. 中国农业科学, 2019, 52(23): 4415-4422. |
| [8] | 王小蕾,林健,杨志远,刘立新,段会娟,程慧敏,赵际成,潘洁,刘月焕. 鸭坦布苏病毒病灭活疫苗血清学试验与免疫攻毒保护试验的相关性[J]. 中国农业科学, 2019, 52(23): 4423-4428. |
| [9] | 葛廷,黄雪,谢让金. 柑橘CitPG34的克隆、定位与表达分析[J]. 中国农业科学, 2019, 52(19): 3404-3416. |
| [10] | 宁越,米雪,陈星伊,邵建航,昝林森. SMAD1基因的沉默和过表达及对秦川牛原代成肌细胞生肌的影响[J]. 中国农业科学, 2019, 52(10): 1818-1829. |
| [11] | 刘超,王玲利,吴頔,党江波,尚维,郭启高,梁国鲁. 枇杷叶片发育基因EjGRF5与启动子克隆及其在不同倍性枇杷中的表达[J]. 中国农业科学, 2018, 51(8): 1598-1606. |
| [12] | 阎依超,万春雁,古咸彬,郭成宝,陈月红,高志红. 过量表达RdreB1BI对草莓果实品质及相关基因的影响[J]. 中国农业科学, 2018, 51(7): 1353-1367. |
| [13] | 刘芳,肖钢,官春云. GT和GATA转录因子对甘蓝型油菜BnA5.FAD2和 BnC5.FAD2启动子功能的调控[J]. 中国农业科学, 2018, 51(24): 4603-4614. |
| [14] | 蒲艳,刘超,李继洋,阿尔祖古丽·塔什,胡燕,刘晓东. 番茄U6启动子的克隆及CRISPR/Cas9基因编辑体系的建立[J]. 中国农业科学, 2018, 51(2): 315-326. |
| [15] | 孔佑宾,李喜焕,张彩英. 大豆紫色酸性磷酸酶基因GmPAP4启动子结构与活性分析[J]. 中国农业科学, 2017, 50(3): 582-590. |
|
||