













中国农业科学 ›› 2020, Vol. 53 ›› Issue (23): 4727-4737.doi: 10.3864/j.issn.0578-1752.2020.23.001
衡燕芳1( ),李健2,王峥2,陈卓1,何航2,邓兴旺2,马力耕1(
),李健2,王峥2,陈卓1,何航2,邓兴旺2,马力耕1( )
)
收稿日期:2020-09-20
接受日期:2020-10-30
出版日期:2020-12-01
发布日期:2020-12-09
通讯作者:
马力耕
作者简介:衡燕芳,E-mail: 基金资助:
HENG YanFang1( ),LI Jian2,WANG Zheng2,CHEN Zhuo1,HE Hang2,DENG XingWang2,MA LiGeng1(
),LI Jian2,WANG Zheng2,CHEN Zhuo1,HE Hang2,DENG XingWang2,MA LiGeng1( )
)
Received:2020-09-20
Accepted:2020-10-30
Online:2020-12-01
Published:2020-12-09
Contact:
LiGeng MA
摘要:
【目的】从小麦隐性核雄性不育突变体ms1与十倍体长穗偃麦草异附加系中,克隆雄性育性恢复基因ThMs1,解析该基因的表达模式、编码蛋白的生物化学活性和生物学功能,鉴定新的小麦ms1育性恢复基因,从而更好地应用于小麦杂交育种。【方法】通过基因组原位杂交(genomic in situ hybridization,GISH),鉴定ms1与十倍体长穗偃麦草异附加系中小麦外源的染色体类型;通过同源克隆法在小麦-十倍体长穗偃麦草异附加系中克隆雄性育性基因ThMs1,并稳定转化小麦ms1进行基因功能互补验证;利用RT-PCR及实时荧光定量PCR分析检测基因的表达模式;进一步通过RNA原位杂交分析基因的组织细胞特异性表达特性;利用特异性抗体进行Western blot检测ThMs1蛋白在体内的表达情况;经SignalP软件分析预测蛋白结构;通过蛋白-脂质结合活性分析检测ThMs1蛋白是否具有脂类分子结合活性。【结果】证实小麦ms1与十倍体长穗偃麦草异附加系细胞中含有偃麦草4Ag染色体;从小麦-十倍体长穗偃麦草异附加系中克隆了雄性育性基因ThMs1,遗传转化功能互补试验证实ThMs1能够完全恢复小麦ms1突变体的雄性不育表型,基因的聚类分析表明MS1只存在于禾本科植物中;通过RT-PCR和实时荧光定量PCR检测发现ThMs1在减数分裂期的花药中特异表达,RNA原位杂交分析证实ThMs1在小麦小孢子发生过程中特异表达;Western blot检测发现ThMs1在小麦减数分裂期的花药中表达;对ThMs1蛋白氨基酸序列进行结构预测发现,该蛋白具有推测的脂类结合分子结构域,ThMs1蛋白脂类分子结合试验表明ThMs1蛋白特异结合磷脂酸和磷酸化的磷脂酰肌醇。【结论】克隆了一个新的来自十倍体长穗偃麦草、可恢复小麦隐性核雄性不育突变体ms1雄性育性的ThMs1,该基因与小麦Ms1具有相似的分子结构、时空表达模式和脂结合活性,导致2个蛋白在小麦中也具有相似的生物学功能,ThMs1可以替代普通小麦Ms1的功能调控小麦花粉育性,该结果为利用小麦ms1通过分子设计建立小麦杂交育种体系(新一代杂交育种体系)提供了一个新的育性恢复基因。
衡燕芳,李健,王峥,陈卓,何航,邓兴旺,马力耕. 十倍体长穗偃麦草雄性育性基因ThMs1的克隆、表达及功能分析[J]. 中国农业科学, 2020, 53(23): 4727-4737.
HENG YanFang,LI Jian,WANG Zheng,CHEN Zhuo,HE Hang,DENG XingWang,MA LiGeng. Cloning, Expression and Functional Analysis of a Male Fertility Gene ThMs1 in Bread Wheat[J]. Scientia Agricultura Sinica, 2020, 53(23): 4727-4737.
附表1
本研究所用引物"
| 引物 Primer | 序列 Sequence (5'→3') | 目的 Intention |
|---|---|---|
| ThMs1-F | AGATCCCGGCGCCTGCTGCTC | ThMs1扩增引物 PCR primer of ThMs1 |
| ThMs1-R | CGCAGGAGCTGTAGAGCGTGAGGA | |
| BF | TAGCCATCTTTGATCAATGAGC | 4Ag染色体鉴定 Analysis of 4Ag chromosome |
| BR | TGATGAAAGAGCTAGGTGATAGTTG | |
| TF1 | CGCCAGGGTTTTCCCAGTCACGAC | 转基因鉴定 Analysis of transgenic lines |
| TR1 | TACAATGGCTAGTAGAGATTTC | |
| TF2 | ATACGTTTCCTGCTACAGATTTGAGG | |
| TR2 | AAGCAGTGCCGCCAGAGGATCAACGC | |
| TF3 | TTTGCGTTCTGCTGATGATGTG | |
| TR3 | CTCAATTGTCCTTTAGACCATGTCTAAC | |
| TaACTIN-QF | TTCCGTTGCCCTGAGGTCC | RT-PCR和实时荧光定量PCR RT-PCR and real time PCR |
| TaACTIN-QR | TGATCTTCATGCTGCTTGGTGC | |
| Ms1-QF | ACATCATCCTCTGAGTCGCG | |
| Ms1-QR | GACCACGCAAACACGTACG | |
| ThMs1-QF | TCCCGGCGCCTGCTGCTC | |
| ThMs1-QR | CGGCAGAGGCAGGGGACG | |
| Ms1-ISH-F | aagcttGAGCGAGGGAGAGAGAGACC | RNA原位杂交 In situ hybridization |
| Ms1-ISH-F | gaattcATCACATAGCATCAGTGGTTC | |
| ThMs1-ISH-F | aagcttCTAGCGAGCGAGCGAGAGG | |
| ThMs1-ISH-R | gaattcCAACTGGACGGACCATGGC | |
| ThMs1-SB-F1 | TGAGATATACGGAGCGATTTAG | Southern杂交 Southern blot |
| ThMs1-SB-R1 | AGCACGGCAAGCTTTTGCTCTG | |
| His-F | aattcCATCATCATCATCATCACTGActgca | MBP-His载体构建 Vector construction of MBP-His |
| His-R | gTCAGTGATGATGATGATGATGg | |
| Ms1s-His-F | gagggaaggatttcagaattcCAGCCGGGGGCGCCGTGC | MBP-Ms1-His载体构建Vector construction of MBP-Ms1-His |
| Ms1s-His-R | cagtgccaagcttgcctgcagTCAGTGATGATGATGATGATGGGCCGCCTTGGACGGCG | |
| ThMs1s-His-F | gagggaaggatttcagaattcGCGTTCGGGCCGCAGC | MBP-ThMs1-His载体构建 Vector construction of MBP-ThMs1-His |
| ThMs1s-His-R | cagtgccaagcttgcctgcagtcaGTGATGATGATGATGATGGAAGAAGGCCGCCTTGGACG |
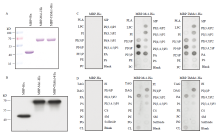
图5
Ms1和ThMs1蛋白体外结合磷脂类分子 A、B:MBP-His、MBP-Ms1-His、MBP-ThMs1-His蛋白的纯化和检测;MBP-His、MBP-Ms1-His、MBP-ThMs1-His蛋白覆膜:脂膜P-6001(C)和脂膜P-6002(D)。CL:心磷脂;CS:胆固醇;DAG:二酰甘油;LPA:溶血磷脂酸;LPC:溶血磷脂酰胆碱;PE:磷脂酰乙醇胺;PG:磷脂酰甘油;PI:磷脂酰肌醇;PI(3)P:磷脂酰肌醇-3-磷酸;PI(4)P:磷脂酰肌醇-4-磷酸;PI(5)P:磷脂酰肌醇-5-磷酸;PI(3,4)P2:磷脂酰肌醇-3,4-二磷酸;PI(3,5)P2:磷脂酰肌醇-3,5-二磷酸酯;PI(4,5)P2:磷脂酰肌醇-4,5-二磷酸;PI(3,4,5)P3:磷脂酰肌醇-3,4,5-三磷酸;PS:磷脂酰丝氨酸;SM:鞘磷脂;S1P:鞘氨醇-1-磷酸;TAG:三酰甘油"
| [1] |
TILMAN D, CASSMAN K G, MATSON P A, NAYLOR R, POLASKY S . Agricultural sustainability and intensive production practices. Nature, 2002,418:671-677.
doi: 10.1038/nature01014 pmid: 12167873 |
| [2] |
FOLEY J A, RAMANKUTTY N, BRAUMAN K A, CASSIDY E S, GERBER J S, JOHNSTON M, MUELLER N D, O’CONNELL C, RAY D K, WEST P C, BALZER C, BENNETT E M, CARPENTER S R, HILL J, MONFREDA C, POLASKY S, ROCKSTRÖM J, SHEEHAN J, SIEBERT S, TILMAN D, ZAKS D P M . Solutions for a cultivated planet. Nature, 2011,478:337-342.
doi: 10.1038/nature10452 pmid: 21993620 |
| [3] |
IWGSC. A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science, 2014,345:1251788.
doi: 10.1126/science.1251788 pmid: 25035500 |
| [4] |
CHANG Z Y, CHEN Z F, WANG N, XIE G, LU J W, YAN W, ZHOU J L, TANG X Y, DENG X W . Construction of a male sterility system for hybrid rice breeding and seed production using a nuclear male sterility gene. Proceedings of the National Academy of Sciences of USA, 2016,113:14145-14150.
doi: 10.1073/pnas.1613792113 |
| [5] |
邓兴旺, 王海洋, 唐晓艳, 周君莉, 陈浩东, 何光明, 陈良碧, 许智宏 . 杂交水稻育种将迎来新时代. 中国科学: 生命科学, 2013,43:864-868.
doi: 10.1360/052013-299 |
|
DENG X W, WANG H Y, TANG X Y, ZHOU J L, CHEN H D, HE G M, CHEN L B, XU Z H . Hybrid rice breeding welcomes a new era of molecular crop design. Scientia Sinica Vitae, 2013,43:864-868. (in Chinese)
doi: 10.1360/052013-299 |
|
| [6] |
CEOLONI C, FORTE P, KUZMANOVIĆ L, TUNDO S, MOSCETTI I, VITA D P, VIRILI M E, OVIDIO R D . Cytogenetic mapping of a major locus for resistance to Fusarium head blight and crown rot of wheat onThinopyrum elongatum 7EL and its pyramiding with valuable genes from a Th. ponticum homoeologous arm onto bread wheat 7DL. Theoretical and Applied Genetics, 2017,130(10):2005-2024.
doi: 10.1007/s00122-017-2939-8 pmid: 28656363 |
| [7] |
ZHANG X Y, LI Z S, CHEN S Y . Production and identification of three 4Ag (4D) substitution lines of Triticum aestivum-Agropyron: relative transmission rate of alien chromosomes. Theoretical and Applied Genetics, 1992,83:707-714.
doi: 10.1007/BF00226688 pmid: 24202744 |
| [8] |
WANG S W, WANG C Y, WANG Y Z, WANG Y J, CHEN C H, JI W Q . Molecular cytogenetic identification of two wheat-Thinopyrum ponticum substitution lines conferring stripe rust resistance. Molecular Breeding, 2019,39:143-153.
doi: 10.1007/s11032-019-1053-9 |
| [9] |
KLINDWORTH D L, WILLIAMS N D, MAAN S S . Chromosomal location of genetic male sterility genes in four mutants of hexaploid wheat. Crop Science, 2002,42:1447-1450.
doi: 10.2135/cropsci2002.1447 |
| [10] | MCINTOSH R A, YAMAZAKI Y, DUBCOVSKY J, ROGERS J, MORRIS C, APPELS R, XIA X C . Catalogue of gene symbols for wheat// Proceedings of the 12th International Wheat Genetics Symposium. Yokohama, Japan, 2013. |
| [11] |
NI F, QI J, HAO Q, LYU B, LUO M, WANG Y, CHEN F, WANG S, ZHANG C, EPSTEIN L, ZHAO X, WANG H, ZHANG X, CHEN C, SUN L, FU D . Wheat Ms2 encodes for an orphan protein that confers male sterility in grass species. Nature Communications, 2017,8:15121-15132.
doi: 10.1038/ncomms15121 pmid: 28452349 |
| [12] |
XIA C, ZHANG L C, ZOU C, GU Y Q, DUAN J L, ZHAO G Y, WU J J, LIU Y, FANG X H, GAO L F, JIAO Y N, SUN J Q, PAN Y H, LIU X, JIA J Z, KONG X Y . A TRIM insertion in the promoter of Ms2 causes male sterility in wheat. Nature Communications, 2017,8:15407-15416.
doi: 10.1038/ncomms15407 pmid: 28497807 |
| [13] |
WANG Z, LI J, CHEN S X, HENG Y F, CHEN Z, YANG J, ZHOU K J, PEI J W, HE H, DENG X W, MA L G . Poaceae-specific MS1 encodes a phospholipid-binding protein for male fertility in bread wheat. Proceedings of the National Academy of Sciences of USA, 2017,114:12614-12619.
doi: 10.1073/pnas.1715570114 |
| [14] |
TUCKER E J, BAUMANU U, KOUIDRI A, SUCHECKI R, BAES M, GARCIA M, OKADA T, DONG C, WU Y, SANDHU A, CIGAN A M, WHITFORD R . Molecular identification of the wheat male fertility gene Ms1 and its prospects for hybrid breeding. Nature Communication, 2017,8:869-879.
doi: 10.1038/s41467-017-00945-2 |
| [15] |
PALLOTTA M A, WARNER P, KOUIDRI A, TUCKER E J, MATHIEU B, SUCHECKI R, WATSON-HAIGH N, OKADA T, GARCIA M, SANDHU A, SINGH M, WOLTERS P, ALBERTSEN M C, CIGANA M, BAUMANN U, WHITFORD R . Wheat ms5 male-sterility is induced by recessive homoeologous A and D genome non-specific lipid transfer proteins. The Plant Journal, 2019,99:673-685.
doi: 10.1111/tpj.14350 pmid: 31009129 |
| [16] | LIU L, LUO Q, LI H, LI B, LI Z, ZHENG Q . Physical mapping of the blue-grained gene from Thinopyrum ponticum chromosome 4Ag and development of blue-grain-related molecular markers and a FISH probe based on SLAF-seq technology. Theoretical and Applied Genetics, 2018,122:1007-1017. |
| [17] |
刘成, 韩冉, 汪晓璐, 宫文萍, 程敦公, 曹新有, 刘爱峰, 李豪圣, 刘建军 . 小麦远缘杂交现状、抗病基因转移及利用研究进展. 中国农业科学, 2020,53(7):1287-1308.
doi: 10.3864/j.issn.0578-1752.2020.07.001 |
|
LIU C, HAN R, WANG X L, GONG W P, CHENG D G, CAO X Y, LIU A F, LI H S, LIU J J . Research progress of wheat wild hybridization, disease resistance genes transfer and utilization. Scientia Agricultura Sinica, 2020,53(7):1287-1308. (in Chinese)
doi: 10.3864/j.issn.0578-1752.2020.07.001 |
|
| [18] |
ZHANG W, CAO Y P, ZHANG M Y, ZHU X W, REN S F, LONG Y M, GYAWALI Y, CHAO S, XU S, CAI X W . Meiotic homoeologous recombination-based alien gene introgression in the genomics era of wheat. Crop Science, 2017,57:1189-1198.
doi: 10.2135/cropsci2016.09.0819 |
| [19] |
KUZMANOVIC L, MANDALÀ G, TUNDO S, CIORBA R, FRANGELLA M, RUGGERI R, ROSSINI F, GEVI F, RINALDUCCI S, CEOLONI C . Equipping durum wheat-Thinopyrum ponticum recombinant lines with a Thinopyrum elongatum major QTL for resistance to fusarium diseases through a cytogenetic strategy. Frontiers in Plant Science, 2019,10:1324.
doi: 10.3389/fpls.2019.01324 pmid: 31695716 |
| [20] |
ZHAO J, HAO W W, TANG C G, YAO H, LI B C, ZHENG Q, LI Z S, ZHANG X Y . Plasticity in Triticeae centromere DNA sequences: a wheat 3 tall wheatgrass (decaploid) model. The Plant Journal, 2019,100:314-327.
doi: 10.1111/tpj.14444 pmid: 31259444 |
| [21] |
SHITSUKAWA N, TAHIRA C, KASSAI K, HIRABAYASHI K, SHIMIZU T, TAKUMI S, MOCHIDA K, KAWAURA K, OGIHARA Y, MURAIA K . Genetic and epigenetic alteration among three homoeologous genes of a class E MADS Box gene in hexaploid wheat. The Plant Cell, 2007,19:1723-1737.
doi: 10.1105/tpc.107.051813 pmid: 17586655 |
| [22] |
BENNING C . Mechanisms of lipid transport involved in organelle biogenesis in plant cells. Annual Review of Cell and Developmental Biology, 2009,25:71-91.
doi: 10.1146/annurev.cellbio.042308.113414 pmid: 19572810 |
| [23] | DOWLER S, KULAR G, ALESSI D R . Protein lipid overlay assay. Science’s Stke, 2002,129:16-26. |
| [24] |
TESTER M, LANGRIDGE P . Breeding technologies to increase crop production in a changing world. Science, 2010,327:818-822.
doi: 10.1126/science.1183700 pmid: 20150489 |
| [25] |
LI S, YANG D, ZHU Y . Characterization and use of male sterility in hybrid rice breeding. Journal of Integrative Plant Biology, 2007,49:791-804.
doi: 10.1111/j.1744-7909.2007.00513.x |
| [26] |
FREEMAN G F . Heredity of quantitative characters in wheat. Genetics, 1919,4:1-93.
pmid: 17245919 |
| [27] | SINGH S K, CHATRATH R, MISHRA B . Perspective of hybrid wheat research: A review. Indian Journal of Agricultural Sciences, 2010,80:1013-1027. |
| [28] |
WHITFORD R, FLEURY D, REIF J C, GARCIA M, OKADA T, KORZUN V, LANGRIDGE P . Hybrid breeding in wheat: Technologies to improve hybrid wheat seed production. Journal of Experimental Botany, 2013,64:5411-5428.
doi: 10.1093/jxb/ert333 |
| [29] |
SINGH S P, SINGH S P, PANDEY T, SINGH R R, SAWANT S V . A novel male sterility-fertility restoration system in plants for hybrid seed production. Scientific Reports, 2015,5:11274-11288.
doi: 10.1038/srep11274 pmid: 26073981 |
| [30] |
KIM Y J, ZHANG D . Molecular control of male fertility for crop hybrid breeding. Trends in Plant Science, 2018,23:53-65.
doi: 10.1016/j.tplants.2017.10.001 pmid: 29126789 |
| [31] |
FRANCKOWIAK J D, MAAN S S, WILLIAMS N D . A proposal for hybrid wheat utilizing Aegilops squarrosa L. cytoplasm. Crop Science, 1976,16:725-728.
doi: 10.2135/cropsci1976.0011183X001600050033x |
| [32] | WILSON J A . Hybrid wheat breeding and commercial seed development. Plant Breeding Reviews, 1984,2:303-319. |
| [33] |
PEREZ-PRAT E, VAN LOOKEREN CAMPAGNE M M . Hybrid seed production and the challenge of propagating male-sterile plants. Trends in Plant Science, 2002,7:199-203.
doi: 10.1016/s1360-1385(02)02252-5 pmid: 11992824 |
| [34] |
成功海, 龚德平, 郭西陵, 邱文兵, 何庆虎 . C49S温光型两系杂种小麦在江汉平原生产应用中的几个问题. 麦类作物学报, 2005,25:147-148.
doi: 10.7606/j.issn.1009-1041.2005.04.168 |
|
CHENG G H, GONG D P, GUO X L, QIU W B, HE Q H . Problems of the hybrid with Chongqing thermo-photo-sensitive male sterility wheat C49S in the plain of Jianghan. Mailei Zuowu Xuebao, 2005,25:147-148. (in Chinese)
doi: 10.7606/j.issn.1009-1041.2005.04.168 |
|
| [35] |
SINGH S P, SRIVASTAVA R, KUMAR J . Male sterility systems in wheat and opportunities for hybrid wheat development. Acta Physiologiae Plantarum, 2015,37:1713-1720.
doi: 10.1007/s11738-014-1713-7 |
| [36] |
SONG S, WANG T, LI Y, HU J, KAN R, QIU M, DENG Y, LIU P, ZHANG L, DONG H, LI C, YU D, LI X, YUAN D, YUAN L, LI L . A novel strategy for creating a new system of third-generation hybrid rice technology using a cytoplasmic sterility gene and a genic male-sterile gene. Plant Biotechnology Journal, 2020: 1-10.
doi: 10.1111/j.1467-7652.2008.00392.x pmid: 19121104 |
| [1] | 唐华苹,陈黄鑫,李聪,苟璐璐,谭翠,牟杨,唐力为,兰秀锦,魏育明,马建. 基于55K SNP芯片的普通小麦穗长非条件和条件QTL分析[J]. 中国农业科学, 2022, 55(8): 1492-1502. |
| [2] | 张勇,阎俊,肖永贵,郝元峰,张艳,徐开杰,曹双河,田宇兵,李思敏,闫俊良,张赵星,陈新民,王德森,夏先春,何中虎. 中麦895高产稳产优质特性遗传解析[J]. 中国农业科学, 2021, 54(15): 3158-3167. |
| [3] | 刘海英,冯必得,茹振钢,陈向东,黄培新,邢晨涛,潘茵茵,甄俊琦. BNS和BNS366小麦雄性不育与内源激素的关系[J]. 中国农业科学, 2021, 54(1): 1-18. |
| [4] | 张晓,李曼,刘大同,江伟,张勇,高德荣. 扬麦系列品种品质性状分析及育种启示[J]. 中国农业科学, 2020, 53(7): 1309-1321. |
| [5] | 战帅帅,白璐,谢磊,夏先春,任毅,吕文娟,曲延英,耿洪伟. 小麦阿拉伯木聚糖阿魏酸酰基转移酶基因的克隆与功能标记开发[J]. 中国农业科学, 2018, 51(19): 3639-3650. |
| [6] | 张福彦,陈锋,程仲杰,杨保安,范家霖,陈晓杰,张建伟,陈云堂,崔龙. 小麦TaLox-B等位变异对脂肪氧化酶活性和面粉色泽的影响[J]. 中国农业科学, 2017, 50(8): 1370-1377. |
| [7] | 董磊,董晴,张文利,胡晓龙,王洪刚,王玉海. 拟斯卑尔脱山羊草的FISH核型分析[J]. 中国农业科学, 2017, 50(8): 1378-1387. |
| [8] | 辛明明,彭惠茹,倪中福,姚颖垠,孙其信. 小麦耐热性的生理遗传研究进展[J]. 中国农业科学, 2017, 50(5): 783-791. |
| [9] | 张润琪,付凯勇,李超,祖赛超,李春艳,李诚. 磷素对小麦(Triticum aestivum L.)淀粉粒微观特性的影响及其形成机理[J]. 中国农业科学, 2017, 50(22): 4235-4246. |
| [10] | 时佳,翟胜男,刘金栋,魏景欣,白璐,高文伟,闻伟锷,何中虎,夏先春,耿洪伟. 普通小麦籽粒过氧化物酶活性全基因组关联分析[J]. 中国农业科学, 2017, 50(21): 4212-4227. |
| [11] | 刘新伦,王超,牛丽华,刘志立,张录德,陈春环,张荣琦,张宏,王长有,王亚娟,田增荣,吉万全. 普通小麦-十倍体长穗偃麦草衍生新品种抗赤霉病基因的分子鉴别[J]. 中国农业科学, 2017, 50(20): 3908-3917. |
| [12] | 茹京娜,于太飞,陈隽,陈明,周永斌,马有志,徐兆师,闵东红. 小麦锌指转录因子TaDi19A对低温的响应及其互作蛋白的筛选[J]. 中国农业科学, 2017, 50(13): 2411-2422. |
| [13] | 王坤杨,张伟,张双喜,刘宏伟,王轲,杜丽璞,林志珊,叶兴国. 化学杀雄剂SQ-1和阿拉伯葡聚糖蛋白对小麦品种间杂交及远缘杂交成胚率的影响[J]. 中国农业科学, 2016, 49(24): 4824-4832. |
| [14] | 刘自成,苗丽丽,王景一,杨德龙,毛新国,景蕊莲. 普通小麦转录因子基因TaWRKY35的克隆及功能分析[J]. 中国农业科学, 2016, 49(12): 2245-2254. |
| [15] | 熊淑萍,吴克远,王小纯,张捷,杜盼,吴懿鑫,马新明. 不同氮效率基因型小麦根系吸收特性与氮素利用差异的分析[J]. 中国农业科学, 2016, 49(12): 2267-2279. |
|
||