













中国农业科学 ›› 2023, Vol. 56 ›› Issue (3): 529-548.doi: 10.3864/j.issn.0578-1752.2023.03.011
收稿日期:2022-04-30
接受日期:2022-07-22
出版日期:2023-02-01
发布日期:2023-02-14
通信作者:
王春晓,Tel:18111908483;E-mail:cxwang@gzu.edu.cn
联系方式:
王春晓,Tel:18111908483;E-mail:cxwang@gzu.edu.cn
基金资助:
WANG ChunXiao1( ), YU JunZhu1, ZHOU WenYa1, XU YinHu2
), YU JunZhu1, ZHOU WenYa1, XU YinHu2
Received:2022-04-30
Accepted:2022-07-22
Published:2023-02-01
Online:2023-02-14
摘要:
葡萄皮上天然存在着非酿酒酵母属酵母(non-Saccharomyces),主要在葡萄酒浸渍和发酵初期发挥作用,近年来非酿酒酵母属酵母在葡萄酒发酵中的应用受到越来越多的关注。相对于酿酒酵母,非酿酒酵母属酵母在酒精发酵中具有较弱的发酵力,可将还原糖转化为乙醇及其他代谢副产物,是生产复杂风味和低酒度葡萄酒的潜在优良酵母。不同非酿酒酵母属酵母菌种在葡萄酒发酵应用中具有不同的代谢特征,选择具有一定特征的优良非酿酒酵母属酵母应用于发酵中,可以提高葡萄酒的特色化品质。本研究在总结商业化非酿酒酵母属酵母的种类、酿造特点和应用方式的基础上,重点综述了不同非酿酒酵母属酵母对葡萄酒颜色、香气、口感和安全健康4个方面的积极作用、代谢机理和研究热点:具有高产酸、多糖、胞外丙酮酸及低吸附性等特性的非酿酒酵母属酵母可通过不同代谢机理促进葡萄酒颜色的稳定;不同非酿酒酵母属酵母通过低产乙醇、乙醛、降低挥发性酚类,高产乙酸乙酯、乙酸酯类化合物、乙酯类化合物、高级醇、与萜烯或硫醇释放相关的酶类等途径促进葡萄酒果味香气的提升,增加香气复杂性;非酿酒酵母属酵母通过高产甘油、多糖和乳酸,降解苹果酸等方式调节葡萄酒的口感特征;非酿酒酵母属酵母作为生物防治剂可以降低葡萄酒酿造中二氧化硫的用量,通过代谢降解作用减少有毒化合物,提升葡萄酒的安全质量。本文进一步解析了非酿酒酵母属酵母的基因组和微卫星位点分析研究现状,探讨了目前非酿酒酵母属酵母葡萄酒发酵应用研究的主要接种策略,提出了未来研究仍需关注的热点方向,为非酿酒酵母属酵母在葡萄酒酒精发酵中的应用研究提供理论参考。
王春晓, 俞俊竹, 周文亚, 许引虎. 非酿酒酵母属酵母的葡萄酒发酵应用研究进展[J]. 中国农业科学, 2023, 56(3): 529-548.
WANG ChunXiao, YU JunZhu, ZHOU WenYa, XU YinHu. Research Progress on the Application of Non-Saccharomyces During Wine Fermentation[J]. Scientia Agricultura Sinica, 2023, 56(3): 529-548.
表1
已经商业化应用于葡萄酒酿造领域的非酿酒酵母属酵母"
| 菌种名称 Species | 葡萄酒酿造应用特点 Winemaking application characteristics | 产品名称a Product | 生产商 Producer | 菌剂加工类型 Yeast processing type |
|---|---|---|---|---|
| 威克克鲁维酵母 Kluyveromyces wickerhamii | 产真菌毒素抑制败坏菌生长 Production of a mycotoxin inhibiting the growth of spoilage microorganisms | ENARTIS FERM BRETT OUT K[ | 意大利Enartis Italy Enartis | 活性干酵母 Active dry yeast |
| 耐热克鲁维酵母 Lachancea thermotolerans | 产乳酸[ Production of lactic acid | CVE-7 | 中国安琪Angel China Angel | 活性干酵母 Active dry yeast |
| 产乳酸增加酸度,低产乙醇 Production of lactic acid to increase acidity and low production of ethanol | LEVULIA Alcomeno | 意大利AEB Italy AEB | 活性干酵母 Active dry yeast | |
| 高产乳酸增酸 High production of lactic acid to increase acidity | LAKTIA | 葡萄牙Proenol Portugal Proenol | 活性干酵母 Active dry yeast | |
| 增加酸度 Increase acidity | Concerto[ | 丹麦CHR Hansen Denmark CHR Hansen | 活性干酵母 Active dry yeast | |
| 增加酸度和清新感 Increase acidity and freshness | LEVEL2 LAKTIA | 加拿大Lallemand Canada Lallemand | 活性干酵母 Active dry yeast | |
| 核果梅奇酵母 Metschnikowia fructicola | 葡萄采收期或冷浸渍期间抑制有害微生物生长 Inhibit the growth of harmful microorganisms during grape harvest or cold maceration | GAÏA | 加拿大Lallemand Canada Lallemand | 活性干酵母 Active dry yeast |
| 美极梅奇酵母 Metschnikowia pulcherrima | 增加品种香气,与酿酒酵母顺序接种使用 Increase the aroma of grapevarieties and inoculate with Saccharomyces cerevisiae in sequence | LEVULIA PULCHERRIMA | 意大利AEB Italy AEB | 活性干酵母 Active dry yeast |
| 葡萄采摘后立即使用,用于生物防治 Use immediately after grape picking for biocontrol purposes | PRIMAFLORA VB BIO | 意大利AEB Italy AEB | 活性干酵母 Active dry yeast | |
| 控制葡萄表面的野生菌,减少二氧化硫使用 Control wild bacteria on the surface of grapes and reduce the use of sulfur dioxide | OenofermMProtect | 德国Erbslöeh Germany Erbslöeh | 活性干酵母 Active dry yeast | |
| 生物防治,减少二氧化硫使用 Biological control and reduction of sulfur dioxide use | LEVEL2 GUARDIA | 葡萄牙Proenol Portugal Proenol | 活性干酵母 Active dry yeast | |
| 增加品种香气 Increase the aroma of grape varieties | FLAVIA | 葡萄牙Proenol Portugal Proenol | 活性干酵母 Active dry yeast | |
| 高消耗溶解氧防止氧化,二氧化硫替代品 High consumption of dissolved oxygen to prevent oxidation, sulfur dioxide substitute | LEVEL2 INITIA | 葡萄牙Proenol Portugal Proenol | 活性干酵母 Active dry yeast | |
| 低温浸渍阶段生物防治 Biocontrol at low temperature maceration stages | ZYMAFLORE KHIOMP | 法国Laffort France Laffort | 活性干酵母 Active dry yeast | |
| 增加品种特性 Increase grape variety characteristics | LEVEL2 FLAVIA | 加拿大Lallemand Canada Lallemand | 活性干酵母 Active dry yeast | |
| 美极梅奇酵母+戴尔有孢圆酵母 Metschnikowia pulcherrima + Torulaspora delbrueckii | 生物防治,减少二氧化硫使用 Biological control and reduction of sulfur dioxide use | ZYMAFLORE ÉGIDETDMP | 法国Laffort France Laffort | 活性干酵母 Active dry yeast |
| 克鲁维毕赤酵母 Pichia kluyveri | 增加果味 Increase fruitiness | Footzen[ | 丹麦CHR Hansen Denmark CHR Hansen | 活性干酵母 Active dry yeast |
| 克鲁维毕赤酵母+瑟氏哈萨克 斯坦酵母 P. kluyveri+Kazachstania servazzii | 产生大量香气,生物防治用作二氧化硫替代品 Production of high aroma, and biocontrol is used as a sulphur dioxide substitute | Thrillyeast | 意大利BioEnologia Italy BioEnologia | 黏稠奶油状的液体酵母 Thick and creamy liquid yeast |
| 酵母属+美极梅齐酵母 Saccharomyces + Metschnikowia pulcherrima | 葡萄采摘后立即使用,用于生物防治 Used immediately after grape picking for biocontrol purposes | PRIMAFLORA VR BIO | 意大利AEB Italy AEB | 活性干酵母 Active dry yeast |
| 酵母属+戴尔有孢圆酵母 Saccharomyces + Torulaspora delbrueckii | 增加香气复杂性 Increase aroma complexity | Oenoferm wild & pure F3 | 德国Erbslöeh Germany Erbslöeh | 活性干酵母 Active dry yeast |
| 酿酒酵母+戴尔有孢圆酵母+耐热克鲁维酵母 S. cerevisiae+T. delbrueckii + L. thermotolerans | 增加果香 Increase fruity aroma | Melody[ | 丹麦CHR Hansen Denmark CHR Hansen | 活性干酵母 Active dry yeast |
| 粟酒裂殖酵母 Schizosaccharomyces pombe | 降解苹果酸、葡萄糖酸和赭曲霉毒素,高产甘油、多糖和乙醇,低产乙酸,不产组胺,提升颜色 Degradation of malic acid, gluconic acid and ochratoxin, high production of glycerol, polysaccharides and ethanol, low production of acetic acid, no histamine, color enhancement | Atecrem 12H | 意大利BioEnologia Italy BioEnologia | 黏稠奶油状的液体酵母 Thick and creamy liquid yeast |
| 降低苹果酸 Lower malic acid | PROMALIC | 葡萄牙Proenol Portugal Proenol | 胶囊封装酵母 Capsule encapsulated yeast | |
| 星形假丝酵母 Starmerella bacillaris | 高产甘油,低产乙醇,需无二氧化硫酿造 High production of glycerine, low production of ethanol, no sulphur dioxide required for brewing | Atecrem 11H | 意大利BioEnologia Italy BioEnologia | 黏稠奶油状的液体酵母 Thick and creamy liquid yeast |
| 戴尔有孢圆酵母 Torulaspora delbrueckii | 增加香气复杂性,释放多糖增加口感圆润度 Increases aromatic complexity and releases polysaccharides to increase roundness on the palate | NSD | 中国安琪Angel China Angel | 活性干酵母 Active dry yeast |
| 增加果香和花香,低产挥发酸,增加复杂性 Increase fruit and floral aromas, low yield of volatile acids, increases complexity | EnartisFerm Q Tau | 意大利Enartis Italy Enartis | 活性干酵母 Active dry yeast | |
| 增加香气复杂性 Increase aroma complexity | BIODIVA | 葡萄牙Proenol Portugal Proenol | 活性干酵母 Active dry yeast | |
| 增加花香和品种香气,释放大量甘露糖蛋白增加口感圆润度 Increase floral and varietal aromas and release a large amount of mannose protein to increase roundness on the palate | VINIFERM NSTD | 西班牙Agrovin Spain Agrovin | 活性干酵母 Active dry yeast | |
| 与酿酒酵母结合使用,增加口感长度和香气强度,低产挥发酸 Combination with Saccharomyces cerevisiae for increasing length and aroma intensity, and low volatile acid production | ZYMAFLORE ALPHA | 法国Laffort France Laffort | 活性干酵母 Active dry yeast | |
| 增加品种特性和发酵酯类特性 Increase in grape variety characteristics and fermentation ester characteristics | LEVEL2 BIODIVA | 加拿大Lallemand Canada Lallemand | 活性干酵母 Active dry yeast | |
| 增加香气 Increase aroma | Prelude[ | 丹麦CHR Hansen Denmark CHR Hansen | 活性干酵母 Active dry yeast | |
| 异常威克汉姆酵母 Wickerhamomyces anomalus | 产真菌毒素抑制败坏菌生长 Production of a mycotoxin inhibiting the growth of spoilage microorganisms | ENARTIS FERM BRETT OUT W[ | 意大利Enartis Italy Enartis | 活性干酵母 Active dry yeast |
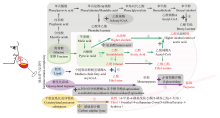
图3
与酵母菌发酵途径关联的香气物质形成机理[9,17,19,28,38⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓-54] ①高产乙醛:日本裂殖酵母、粟酒裂殖酵母、星形假丝酵母;低产乙醛、乙酸或3-羟基丁烷-2-酮:耐热克鲁维酵母、L. fermentati、美极梅奇酵母、特立科拉毕赤酵母、库德毕赤酵母、星形假丝酵母、戴尔有孢圆酵母;②降低乙醇:耐热克鲁维酵母、美极梅奇酵母、星形假丝酵母、戴尔有孢圆酵母;③高产高级醇类化合物(Ehrlich途径、糖代谢合成途径):葡萄酒有孢汉逊酵母、季也蒙毕赤酵母、美极梅奇酵母、发酵毕赤酵母、胶红酵母、日本裂殖酵母;低产高级醇类化合物:有孢汉逊酵母属、星形假丝酵母、粟酒裂殖酵母、接合酵母属;④高产乙酸酯类化合物:加利福尼亚假丝酵母、土星形塞伯林德纳氏酵母、季也蒙有孢汉逊酵母、耐渗透压有孢汉逊酵母、葡萄汁有孢汉逊酵母、有孢汉逊酵母属、好氧哈萨克斯坦酵母、瑟氏哈萨克斯坦酵母、美极梅奇酵母、克鲁维毕赤酵母、特立科拉毕赤酵母、库德毕赤酵母;葡萄酒有孢汉逊酵母通过分枝酸-预苯酸途径高产乙酸酯类化合物;⑤高产乙酸乙酯:季也蒙有孢汉逊酵母、葡萄酒有孢汉逊酵母、葡萄汁有孢汉逊酵母、特立科拉毕赤酵母、库德毕赤酵母、克鲁维毕赤酵母、日本裂殖酵母、拜耳接合酵母;⑥高产乙酯类化合物:有孢汉逊酵母属、葡萄汁有孢汉逊酵母、克鲁维毕赤酵母、库德毕赤酵母、特立科拉毕赤酵母、克拉通覆膜孢酵母、星形假丝酵母、戴尔有孢圆酵母、拜耳接合酵母;⑦高产酶类,释放萜烯类化合物或品种硫醇等:加利福尼亚假丝酵母、铁红假丝酵母、有孢汉逊酵母属、葡萄汁有孢汉逊酵母、耐热克鲁维酵母、美极梅奇酵母、克鲁维毕赤酵母、库德毕赤酵母、胶红酵母、星形假丝酵母、克拉通覆膜孢酵母、戴尔有孢圆酵母"
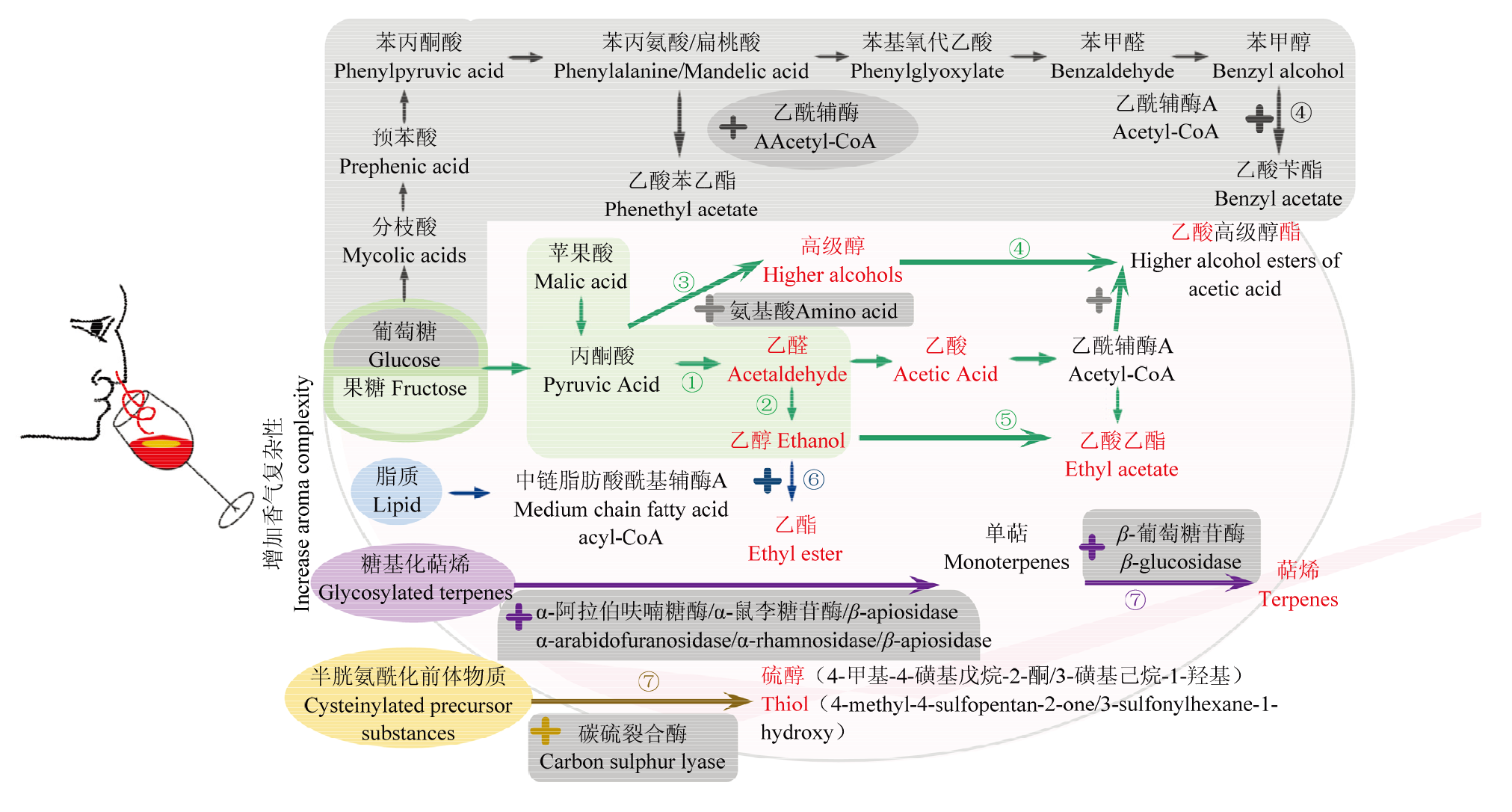
表2
非酿酒酵母属酵母的基因组相关信息"
| 菌种名称1) Species | 基因组大小2) Genome size | 基因组序列(个)2) Genome sequence | 倍性与孢子形成3) Ploidy and sporulation | 微卫星位点分析 Microsatellite locus analysis | ||
|---|---|---|---|---|---|---|
| 加利福尼亚假丝酵母 C. californica | 细胞核:12.32 Mb Nucleus: 12.32 Mb | 2 | 未知 Unknown | 无 None | ||
| 光滑假丝酵母 C. glabrata | 细胞核:12.14—14.56 Mb,13条染色体;线粒体:20 kb Nucleus: 12.14-14.56 Mb, 13 Chromosomes; Mitochondria: 20 kb | 36 | 未知 Unknown | 无 None | ||
| 铁杉假丝酵母 C. railenensis | 无 None | 无 None | 未知 Unknown | 无 None | ||
| 土星形塞伯林德纳氏酵母 C. saturnus | 细胞核:13.43 Mb Nucleus: 13.43 Mb | 1 | 未知 Unknown | 无 None | ||
| 汉氏德巴利氏酵母 D. hansenii | 细胞核:11.46—18.56 Mb,7条染色体;线粒体:30 kb Nucleus: 11.46-18.56 Mb, 7 Chromosomes; Mitochondria: 30 kb | 12 | 单倍体,1个子囊1个孢子,偶尔2个 Haploid, 1 ascus 1 spore, occasionally 2 | 无 None | ||
| 季也蒙有孢汉逊酵母 H. guilliermondii | 细胞核:9.04—9.14 Mb,8—9条染色体 Nucleus: 9.04-9.14 Mb, 8-9 Chromosomes | 2 | 倍性未知,1个子囊4个孢子 Ploidy unknown, 1 ascus 4 spores | 无 None | ||
| 耐渗透压有孢汉逊酵母 H. osmophila | 细胞核:11.46—12.16 Mb Nucleus: 11.46-12.16 Mb | 2 | 倍性未知,1个子囊1—2个孢子 Ploidy unknown, 1 ascus 1-2 spores | 无 None | ||
| 葡萄汁有孢汉逊酵母 H. uvarum | 细胞核:8.1—9.5 Mb,8—9条染色体;线粒体:11 kb Nucleus: 8.1-9.5 Mb, 8-9 Chromosomes; Mitochondria: 11 kb | 10 | 倍性未知可能是二倍体,1个子囊1个 孢子,罕见2个孢子 Unknown ploidy is likely diploid, 1 ascus 1 spore, rare 2 spores | 10个微卫星位点[ Ten microsatellite loci[ | ||
| 葡萄酒有孢汉逊酵母 H. vineae | 细胞核:11.1—11.37 Mb,5条染色体;线粒体:28—45 kb Nucleus: 11.1-11.37 Mb, 5 Chromosomes; Mitochondria: 28-45 kb | 3 | 倍性未知,1个子囊1个孢子,罕见2 个孢子 Ploidy unknown, 1 ascus 1 spore, rare 2 spores | 无 None | ||
| 好氧哈萨克斯坦酵母 K. aerobia | 细胞核:12.09 Mb Nucleus: 12.09 Mb | 1 | 倍性未知,1个子囊1个孢子 Ploidy unknown, 1 ascus 1 spore | 无 None | ||
| 瑟氏哈萨克斯坦酵母 K. servazzii | 细胞核:11.77—12.84 Mb Nucleus: 11.77-12.84 Mb | 4 | 倍性未知,1个子囊1个孢子,偶尔 2—4个孢子 Ploidy unknown, 1 ascus 1 spore, occasionally 2-4 spores | 无 None | ||
| L. fermentati | 细胞核:10.26 Mb,8条染色体 Nucleus: 10.26 Mb, 8 Chromosomes | 2 | 倍性未知,1个子囊1—4个孢子 Ploidy unknown, 1 ascus 1-4 spores | 无 None | ||
| 耐热克鲁维酵母 L. thermotolerans | 细胞核:10.24—10.39 Mb,8条染色体;线粒体:21.9—25.1 kb Nucleus: 10.24-10.39 Mb, 8 Chromosomes; Mitochondria: 21.9-25.1 kb | 3 | 倍性有争议,单倍体或二倍体,1个 子囊1—4个孢子 Ploidy is controversial, haploid or diploid, 1 ascus 1-4 spores | 14个微卫星位点[ Fourteen microsatellite loci [ | ||
| 核果梅奇酵母 M. fructicola | 细胞核:26.13—26.18 Mb Nucleus: 26.13-26.18 Mb | 2 | 倍性未知,1个子囊2个孢子 Ploidy unknown, 1 ascus 2 spores | 无 None | ||
| 美极梅齐酵母 M. pulcherrima | 细胞核:21.77 Mb Nucleus: 21.77 Mb | 1 | 二倍体,1个子囊1—2个孢子 Diploid, 1 ascus 1-2 spores | 无 None | ||
| 季也蒙毕赤酵母 M. guilliermondii | 细胞核:10.59—10.71 Mb;线粒体:31 kb Nucleus: 10.59-10.71 Mb; Mitochondria: 31 kb | 10 | 倍性未知,1个子囊1—4个孢子 Ploidy unknown, 1 ascus 1-4 spores | 3个微卫星位点[ Three microsatellite loci[ | ||
| 发酵毕赤酵母 P. fermentans | 细胞核:10.57 Mb,可能2条染色体 Nucleus: 10.57 Mb, possibly 2 chromosomes | 1 | 倍性未知,1个子囊2—4个孢子 Ploidy unknown, 1 ascus 2-4 spores | 无 None | ||
| 克鲁维毕赤酵母 P. kluyveri | 细胞核:10.96—12.40 Mb;线粒体:43.1 kb Nucleus: 10.96-12.40 Mb; Mitochondria: 43.1 kb | 3 | 二倍体,1个子囊4个孢子 Diploid, 1 ascus 4 spores | 无 None | ||
| 库德毕赤酵母 P. kudriavzevii | 细胞核:7.25—12.94 Mb,5条染色体;线粒体:50 kb Nucleus: 7.25-12.94 Mb, 5 Chromosomes; Mitochondria: 50 kb | 33 | 二倍体,1个子囊1—2个孢子 Diploid, 1 ascus 1-2 spores | 无 None | ||
| 特立科拉毕赤酵母 P. terricola | 细胞核:11.01—14.04 Mb Nucleus: 11.01-14.04 Mb | 6 | 倍性未知,1个子囊1—4个孢子 Ploidy unknown, 1 ascus 1-4 spores | 无 None | ||
| 胶红酵母 R. mucilaginosa | 细胞核:19.04—20.22 Mb;线粒体:47 kb Nucleus: 19.04-20.22 Mb; Mitochondria: 47 kb | 102 | 未知 Unknown | 无 None | ||
| 星形假丝酵母 S. bacillaris | 细胞核:7.27—9.36 Mb,3条染色体;线粒体:23 kb Nucleus: 7.27-9.36 Mb, 3 Chromosomes; Mitochondria: 23 kb | 5 | 倍性未知可能是单倍体,无证据表明 具备形成孢子的能力 Ploidy unknown probably haploid, no evidence of ability to form spores | 十个微卫星位点[ Ten microsatellite loci[ | ||
| 克拉通覆膜孢酵母 S. crataegensis | 细胞核:15.37 Mb Nucleus: 15.37 Mb | 1 | 倍性未知可能是单倍体,偶尔形成子囊, 1个子囊2个孢子 Ploidy unknown probably haploid, occasionally forming ascus, 1 ascus 2 spores | 无 None | ||
| 日本裂殖酵母 S. japonicus | 细胞核:11.54—11.73 Mb;线粒体:80 kb Nucleus: 11.54-11.73 Mb; Mitochondria: 80 kb | 2 | 倍性未知,1个子囊6—8个孢子 Ploidy unknown, 1 ascus 6-8 spores | 无 None | ||
| 粟酒裂殖酵母 S. pombe | 细胞核:12.59—13.15 Mb,3条染色体;线粒体:20 kb Nucleus: 12.59-13.15 Mb, 3 Chromosomes; Mitochondria: 20 kb | 20 | 倍性未知,1个子囊2—4个孢子 Ploidy unknown, 1 ascus 2-4 spores | 无 None | ||
| 戴尔有孢圆酵母 T. delbrueckii | 细胞核:9.00—11.53 Mb,8条染色体;线粒体:28—45 kb Nucleus: 9.00-11.53 Mb, 8 Chromosomes; Mitochondria: 28-45 kb | 77 | 倍性未知可能是二倍体,1个子囊1个 孢子,偶尔2—3个孢子 Ploidy unknown probably diploid, 1 ascus 1 spore, occasionally 2-3 spores | 8个微卫星位点[ Eight microsatellite loci[ | ||
| 异常威克汉姆酵母 W.anomalus | 细胞核:12.72—26.55 Mb Nucleus: 12.72-26.55 Mb | 10 | 单倍体和二倍体,1个子囊1-4个孢子 Haploid and diploid, 1 ascus 1-4 spores | 无 None | ||
| 拜耳接合酵母 Z. bailii | 细胞核:10.27—21.14 Mb,5—13条染色体;线粒体:29.5 kb Nucleus: 10.27-21.14 Mb, 5-13 Chromosomes; Mitochondria: 29.5 kb | 3 | 单倍体和二倍体,1个子囊1—4个孢子 Haploid and diploid, 1 ascus 1-4 spores | 无 None | ||
| [1] |
JOLLY N P, VARELA C, PRETORIUS I S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Research, 2014, 14(2): 215-237. doi: 10.1111/1567-1364.12111.
doi: 10.1111/1567-1364.12111 |
| [2] | KURTZMAN C P, FELL J W, BOEKHOUT T. The Yeasts:A Taxonomic Study. 5th ed. Amsterdam: Elsevier, 2011. |
| [3] |
DRUMONDE-NEVES J, FERNANDES T, LIMA T, PAIS C, FRANCO-DUARTE R. Learning from 80 years of studies: A comprehensive catalogue of non-Saccharomyces yeasts associated with viticulture and winemaking. FEMS Yeast Research, 2021, 21(3): foab017. doi: 10.1093/femsyr/foab017.
doi: 10.1093/femsyr/foab017 |
| [4] | PADILLA B, GIL J V, MANZANARES P. Past and future of non- Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Frontiers in Microbiology, 2016, 7: 411. |
| [5] |
MAS A, PADILLA B, ESTEVE-ZARZOSO B, BELTRAN G, REGUANT C, BORDONS A. Taking advantage of natural biodiversity for wine making: The wildwine project. Agriculture and Agricultural Science Procedia, 2016, 8: 4-9.
doi: 10.1016/j.aaspro.2016.02.002 |
| [6] |
BERBEGAL C, FRAGASSO M, RUSSO P, BIMBO F, GRIECO F, SPANO G, CAPOZZI V. Climate changes and food quality: The potential of microbial activities as mitigating strategies in the wine sector. Fermentation, 2019, 5: 85.
doi: 10.3390/fermentation5040085 |
| [7] | LAIRÓN-PERIS M, PÉREZ-TRAVÉS L, MUÑIZ-CALVO S, GUILLAMÓN J M, HERAS J M, BARRIO E, QUEROL A. Differential contribution of the parental genomes to a S. cerevisiae × S. uvarum hybrid, inferred by phenomic, genomic, and transcriptomic analyses, at different industrial stress conditions. Frontiers Media, 2020, 8: 129. |
| [8] |
ALBERTIN W, ZIMMER A, MIOT-SERTIER C, BERNARD M, COULON J, MOINE V, COLONNA-CECCALDI B, BELY M, MARULLO P, MASNEUF-POMAREDE I. Combined effect of the Saccharomyces cerevisiae lag phase and the non-Saccharomyces consortium to enhance wine fruitiness and complexity. Applied Microbiology and Biotechnology, 2017, 101(20): 7603-7620.
doi: 10.1007/s00253-017-8492-1 |
| [9] |
BENITO S, CALDERÓN F. The influence of non-Saccharomyces species on wine fermentation quality parameters. Fermentation, 2019, 5: 54.
doi: 10.3390/fermentation5030054 |
| [10] |
BORREN E, TIAN B. The important contribution of non- Saccharomyces yeasts to the aroma complexity of wine: A review. Foods, 2020, 10: 13.
doi: 10.3390/foods10010013 |
| [11] |
TAILLANDIER P, LAI Q P, JULIEN-ORTIZ A, BRANDAM C. Interactions between Torulaspora delbrueckii and Saccharomyces cerevisiae in wine fermentation: Influence of inoculation and nitrogen content. World Journal of Microbiology and Biotechnology, 2014, 30(7): 1959-1967.
doi: 10.1007/s11274-014-1618-z |
| [12] |
PRIOR K J, BAUER F F, DIVOL B. The utilisation of nitrogenous compounds by commercial non-Saccharomyces yeasts associated with wine. Food Microbiology, 2019, 79: 75-84.
doi: 10.1016/j.fm.2018.12.002 |
| [13] | ROUDIL L, RUSSO P, BERBEGAL C, ALBERTIN W, SPANO G, CAPOZZI V. Non-Saccharomyces commercial starter cultures: scientific trends, recent patents and innovation in the wine sector. Recent Patents on Food, Nutrition & Agriculture, 2020, 11(1): 27-39. |
| [14] |
唐冲, 成池芳, 许引虎, 段长青, 燕国梁. 耐热克鲁维酵母在葡萄酒发酵中的研究进展. (2022-04-15)[2022-06-25]. 食品科学:1-12. DOI:10.7506/spkx1002-6630-20211223-266.
doi: 10.7506/spkx1002-6630-20211223-266 |
|
TANG C, CHENG C F, XU Y H, DUAN C Q, YAN G L. Research progresses of Lachanceathermotolerans in wine fermentation. (2022-04-15)[2022-06-25]. Food Science:1-12. doi:10.7506/spkx1002-6630-20211223-266. (in Chinese)
doi: 10.7506/spkx1002-6630-20211223-266 |
|
| [15] |
董琦楠, 李莹, 叶冬青, 刘延琳. 耐热克鲁维酵母在葡萄酒酿造中的研究进展. 微生物学通报, 2022, 49(5): 1941-1954. doi: 10.13344/j.microbiol.china.210653.
doi: 10.13344/j.microbiol.china.210653 |
|
DONG Q N, LI Y, YE D Q, LIU Y L. Research progress of Lachancea thermotolerans in winemaking. Microbiology China, 2022, 49(5): 1941-1954. doi: 10.13344/j.microbiol.china.210653. (in Chinese)
doi: 10.13344/j.microbiol.china.210653 |
|
| [16] |
VEJARANO R, GIL-CALDERÓN A. Commercially available non- Saccharomyces yeasts for winemaking: Current market, advantages over Saccharomyces, biocompatibility, and safety. Fermentation, 2021, 7(3): 171.
doi: 10.3390/fermentation7030171 |
| [17] |
LIN M M-H, BOSS P K, WALKER M E, SUMBY K M, JIRANEK V. Influence of Kazachstania spp. on the chemical and sensory profile of red wines. International Journal of Food Microbiology, 2022, 362: 109496.
doi: 10.1016/j.ijfoodmicro.2021.109496 |
| [18] |
GIANVITO P D, ENGLEZOS V, RANTSIOU K, COCOLIN L. Bioprotection strategies in winemaking. International Journal of Food Microbiology, 2022, 364: 109532.
doi: 10.1016/j.ijfoodmicro.2022.109532 |
| [19] |
TUFARIELLO M, FRAGASSO M, PICO CARBAJO J, PANIGHEL A, CASTELLARIN S D, FLAMINI R, GRIECO F. Influence of non-Saccharomyces on wine chemistry: A focus on aroma-related compounds. Molecules, 2021, 26: 644.
doi: 10.3390/molecules26030644 |
| [20] |
CLEMENTE-JIMENEZ J M, MINGORANCE-CAZORLA L, MARTÍNEZ-RODRÍGUEZ S, HERAS-VÁZQUEZ F J L, RODRÍGUEZ- VICO F. Molecular characterization and oenological properties of wine yeasts isolated during spontaneous fermentation of six varieties of grape must. Food Microbiology, 2004, 21(2): 149-155.
doi: 10.1016/S0740-0020(03)00063-7 |
| [21] |
WANG C X, ESTEVE-ZARZOSO B, MAS A. Monitoring of Saccharomyces cerevisiae, Hanseniaspora uvarum, and Starmerella bacillaris (synonym Candida zemplinina) populations during alcoholic fermentation by fluorescence in situ hybridization. International Journal of Food Microbiology, 2014, 191: 1-9.
doi: 10.1016/j.ijfoodmicro.2014.08.014 |
| [22] | 李华, 王华, 袁春龙. 葡萄酒工艺学. 北京: 科学出版社, 2007: 24-27. |
| LI H, WANG H, YUAN C L. Wine Technology. Beijing: Science Press, 2007: 24-27. (in Chinese) | |
| [23] |
王二雷, 刘彦君, 刘静波. 吡喃型花青素类化合物的研究进展. 食品工业科技, 2018, 39(12): 325-333. doi: 10.13386/j.issn1002-0306.2018.12.059.
doi: 10.13386/j.issn1002-0306.2018.12.059 |
|
WANG E L, LIU Y J, LIU J B. Research advances in pyranoanthocyanin compounds. Science and Technology of Food Industry, 2018, 39(12): 325-333. doi: 10.13386/j.issn1002-0306.2018.12.059. (in Chinese)
doi: 10.13386/j.issn1002-0306.2018.12.059 |
|
| [24] |
LI X S, TENG Z J, LUO Z Y, YUAN Y B, ZENG Y Y, HU J, SUN J X, BAI W B. Pyruvic acid stress caused color attenuation by interfering with anthocyanins metabolism during alcoholic fermentation. Food Chemistry, 2022, 372:131251.
doi: 10.1016/j.foodchem.2021.131251 |
| [25] |
张宁, 赵旭, 兰义宾, 石英, 段长青, 吴广枫. 中国东亚种红葡萄酒的颜色特征及酚类组成研究. 中国酿造, 2022, 41(5): 34-41. doi: 10.11882/j.issn.0254-5071.2022.05.007.
doi: 10.11882/j.issn.0254-5071.2022.05.007 |
|
ZHANG N, ZHAO X, LAN Y B, SHI Y, DUAN C Q, WU G F. Color features and phenolic composition of red wines made from East Asian Vitis species native to China. China Brewing, 2022, 41(5): 34-41. doi: 10.11882/j.issn.0254-5071.2022.05.007. (in Chinese)
doi: 10.11882/j.issn.0254-5071.2022.05.007 |
|
| [26] |
BENITO S, PALOMERO F, MORATA A, CALDERÓN F, PALMERO D, SUÁREZ-LEPE J A. Physiological features of Schizosaccharomyces pombe of interest in making of white wines. European Food Research and Technology, 2013, 236(1): 29-36.
doi: 10.1007/s00217-012-1836-2 |
| [27] |
BENITO Á, CALDERÓN F, BENITO S. The combined use of Schizosaccharomyces pombe and Lachancea thermotolerans- Effect on the anthocyanin wine composition. Molecules, 2017, 22(5): 739.
doi: 10.3390/molecules22050739 |
| [28] |
MORATA A, ESCOTT C, BAÑUELOS M A, LOIRA I, DEL FRESNO J M, GONZÁLEZ C, SUÁREZ-LEPE J A. Contribution of non-Saccharomyces yeasts to wine freshness. A review. Biomolecules, 2020, 10(1): 34.
doi: 10.3390/biom10010034 |
| [29] |
赵美, 田秀, 李敏, 高娉娉, 梁丽红, 韩舜愈, 王婧. 粟酒裂殖酵母与酿酒酵母共同接种发酵对‘黑比诺’干红葡萄酒品质的影响. 食品科学, 2021, 42(24): 108-116. doi: 10.7506/spkx1002-6630-20201012-100.
doi: 10.7506/spkx1002-6630-20201012-100 |
|
ZHAO M, TIAN X, LI M, GAO P P, LIANG L H, HAN S Y, WANG J. Effect of mixed culture fermentation with Schizosaccharomyces pombe and Saccharomyces cerevisiae on the quality of ‘Pinot Noir’ dry red wine. Food Science, 2021, 42(24): 108-116. doi: 10.7506/spkx1002-6630-20201012-100. (in Chinese)
doi: 10.7506/spkx1002-6630-20201012-100 |
|
| [30] |
PORTARO L, MAIOLI F, CANUTI V, PICCHI M, LENCIONI L, MANNAZZU I, DOMIZIO P. Schizosaccharomyces japonicus/ Saccharomyces cerevisiae mixed starter cultures: New perspectives for the improvement of Sangiovese aroma, taste, and color stability. LWT, 2022, 156: 113009.
doi: 10.1016/j.lwt.2021.113009 |
| [31] |
VELENOSI M, CRUPI P, PERNIOLA R, MARSICO A D, SALERNO A, ALEXANDRE H, ARCHIDIACONO N, VENTURA M, CARDONE M F. Color stabilization of Apulian red wines through the sequential inoculation of Starmerella bacillaris and Saccharomyces cerevisiae. Molecules, 2021, 26(4): 907.
doi: 10.3390/molecules26040907 |
| [32] | 胡苑. 干红葡萄酒陈酿衍生色素颜色稳定性的研究[D]. 烟台: 烟台大学, 2021. |
| HU Y. Study on the color stability of red wine derived pigments during aging[D]. Yantai: Yantai University, 2021. (in Chinese) | |
| [33] |
HRANILOVIC A, GAMBETTA J M, SCHMIDTKE L, BOSS P K, GRBIN P R, MASNEUF-POMAREDE I, BELY M, ALBERTIN W, JIRANEK V. Oenological traits of Lachancea thermotolerans show signs of domestication and allopatric differentiation. Scientific Reports, 2018, 8(1): 14812.
doi: 10.1038/s41598-018-33105-7 |
| [34] |
MORATA A, ESCOTT C, LOIRA I, DEL FRESNO J M, GONZÁLEZ C, SUÁREZ-LEPE J A. Influence of Saccharomyces and non- Saccharomyces yeasts in the formation of pyranoanthocyanins and polymeric pigments during red wine making. Molecules, 2019, 24(24): 4490.
doi: 10.3390/molecules24244490 |
| [35] |
WINDHOLTZ S, REDON P, LACAMPAGNE S, FARRIS L, LYTRA G, CAMELEYRE M, BARBE J-C, COULON J, THIBON C, MASNEUF-POMARÈDE I. Non-Saccharomyces yeasts as bioprotection in the composition of red wine and in the reduction of sulfur dioxide. LWT, 2021, 149: 111781.
doi: 10.1016/j.lwt.2021.111781 |
| [36] | 李婧. 冰葡萄酒发酵过程中酵母菌群落演潜规律及优良菌株的筛选[D]. 大连: 大连理工大学, 2020. |
| LI J. The dynamic changes of yeast populations during icewine fermentation and screening of the yeast strains[D]. Dalian: Dalian University of Technology, 2020. (in Chinese) | |
| [37] |
HAN X Y, QING X, YANG S Y, LI R L, ZHAN J C, YOU Y L, HUANG W D. Study on the diversity of non-Saccharomyces yeasts in Chinese wine regions and their potential in improving wine aroma by β-glucosidase activity analyses. Food Chemistry, 2021, 360: 129886.
doi: 10.1016/j.foodchem.2021.129886 |
| [38] |
GE Q, GUO C F, YAN Y, SUN X Y, MA T T, ZHANG J, LI C H, GOU C L, YUE T L, YUAN Y H. Contribution of non- Saccharomyces yeasts to aroma-active compound production, phenolic composition and sensory profile in Chinese Vidal icewine. Food Bioscience, 2021, 46: 101152.
doi: 10.1016/j.fbio.2021.101152 |
| [39] |
BINATI R L, LEMOS JUNIOR W J F, LUZZINI G, SLAGHENAUFI D, UGLIANO M, TORRIANI S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. International Journal of Food Microbiology, 2020, 318: 108470.
doi: 10.1016/j.ijfoodmicro.2019.108470 |
| [40] |
SCANSANI S, VAN W N, NADER K B, BEISERT B, BREZINA S, FRITSCH S, SEMMLER H, PASCH L, PRETORIUS I S, VON WALLBRUNN C, SCHNELL S, RAUHUT D. The film-forming Pichia spp. in a winemaker’s toolbox: A simple isolation procedure and their performance in a mixed-culture fermentation of Vitis vinifera L. cv. Gewürztraminer must. International Journal of Food Microbiology, 2022, 365: 109549.
doi: 10.1016/j.ijfoodmicro.2022.109549 |
| [41] |
YAN G L, ZHANG B Q, JOSEPH L, WATERHOUSE A L. Effects of initial oxygenation on chemical and aromatic composition of wine in mixed starters of Hanseniaspora vineae and Saccharomyces cerevisiae. Food Microbiology, 2020, 90:103460.
doi: 10.1016/j.fm.2020.103460 |
| [42] |
HONG M N, LI J, CHEN Y W, QI B Y, HUANG Y P, WU J, YUE H B, TONG Z Q, LIU Y N, WANG F. Impact of mixed non-Saccharomyces yeast during fermentation on volatile aroma compounds of Vidal blanc icewine. LWT, 2021, 145: 111342.
doi: 10.1016/j.lwt.2021.111342 |
| [43] |
孙玉霞, 赵新节. 美极梅奇酵母的代谢特性及其在葡萄酒生产中的应用前景. 食品与发酵工业, 2021, 47(4): 305-311. doi: 10.13995/j.cnki.11-1802/ts.025251.
doi: 10.13995/j.cnki.11-1802/ts.025251 |
|
SUN Y X, ZHAO X J. Metabolic characteristics of Metschnikowia pulcherrima and its application in wine production. Food and Fermentation Industries, 2021, 47(4): 305-311. doi: 10.13995/j.cnki.11-1802/ts.025251. (in Chinese)
doi: 10.13995/j.cnki.11-1802/ts.025251 |
|
| [44] |
LAI Y T, HSIEH C W, LO Y C, LIOU B K, LIN H W, HOU C Y, CHENG K C. Isolation and identification of aroma-producing non-Saccharomyces yeast strains and the enological characteristic comparison in wine making. LWT, 2022, 154: 112653.
doi: 10.1016/j.lwt.2021.112653 |
| [45] | BELDA I, RUIZ J, BEISERT B, NAVASCUÉS E, MARQUINA D, CALDERÓN F, RAUHUT D, BENITO S, SANTOS A. Influence of Torulaspora delbrueckii in varietal thiol (3-SH and 4-MSP) release in wine sequential fermentations. International Journal of FoodMicrobiology, 2017, 257: 183-191. |
| [46] |
SEGUINOT P, BLOEM A, BRIAL P, MEUDEC E, ORTIZ-JULIEN A, CAMARASA C. Analysing the impact of the nature of the nitrogen source on the formation of volatile compounds to unravel the aroma metabolism of two non-Saccharomyces strains. International Journal of Food Microbiology, 2020, 316: 108441.
doi: 10.1016/j.ijfoodmicro.2019.108441 |
| [47] |
SHI W K, WANG J, CHEN F S, ZHANG X Y. Effect of Issatchenkia terricola and Pichia kudriavzevii on wine flavor and quality through simultaneous and sequential co-fermentation with Saccharomyces cerevisiae. LWT, 2019, 116: 108477.
doi: 10.1016/j.lwt.2019.108477 |
| [48] |
李爱华, 王星晨, 彭文婷, 李朔, 陶永胜. 胶红酵母与酿酒酵母混合酒精发酵中酵母生长与糖苷酶活动力学. 西北农业学报, 2018, 27(6): 896-903. doi: 10.7606/j.issn.1004-1389.2018.06.018.
doi: 10.7606/j.issn.1004-1389.2018.06.018 |
|
LI A H, WANG X C, PENG W T, LI S, TAO Y S. Kinetics of biomass and glycosidase activities during mixed alcoholic fermentation of selected Rhodotorula mucilaginosa and Saccharomyces cerevisiae. Acta Agriculturae Boreali-Occidentalis Sinica, 2018, 27(6): 896-903. doi: 10.7606/j.issn.1004-1389.2018.06.018. (in Chinese)
doi: 10.7606/j.issn.1004-1389.2018.06.018 |
|
| [49] |
WANG X C, LI A H, DIZY M, ULLAH N, SUN W X, TAO Y S. Evaluation of aroma enhancement for ‘Ecolly’ dry white wines by mixed inoculation of selected Rhodotorula mucilaginosa and Saccharomyces cerevisiae. Food Chemistry, 2017, 228: 550-559.
doi: 10.1016/j.foodchem.2017.01.113 |
| [50] |
马娜, 王星晨, 孔彩琳, 陶永胜. 胶红酵母与酿酒酵母共发酵对干红葡萄酒香气与色泽的影响. 食品科学, 2021, 42(2): 97-104. doi: 10.7506/spkx1002-6630-20191112-158.
doi: 10.7506/spkx1002-6630-20191112-158 |
|
MA N, WANG X C, KONG C L, TAO Y S. Effect of mixed culture fermentation with Rhodotorula mucilaginosa and Saccharomyces cerevisiae on the aroma and color of red wine. Food Science, 2021, 42(2): 97-104. doi: 10.7506/spkx1002-6630-20191112-158. (in Chinese)
doi: 10.7506/spkx1002-6630-20191112-158 |
|
| [51] |
NISIOTOU A, SGOUROS G, MALLOUCHOS A, NISIOTIS C-S, MICHAELIDIS C, TASSOU C, BANILAS G. The use of indigenous Saccharomyces cerevisiae and Starmerella bacillaris strains as a tool to create chemical complexity in local wines. Food Research International, 2018, 111: 498-508.
doi: 10.1016/j.foodres.2018.05.035 |
| [52] |
ENGLEZOS V, RANTSIOU K, CRAVERO F, TORCHIO F, POLLON M, FRACASSETTI D, ORTIZ-JULIEN A, GERBI V, ROLLE L, COCOLIN L. Volatile profile of white wines fermented with sequential inoculation of Starmerella bacillaris and Saccharomyces cerevisiae. Food Chemistry, 2018, 257: 350-360.
doi: 10.1016/j.foodchem.2018.03.018 |
| [53] |
LEE P R, SAPUTRA A, YU B, CURRAN P, LIU S Q. Effects of pure and mixed-cultures of Saccharomyces cerevisiae and Williopsis saturnus on the volatile profiles of grape wine. Food Biotechnology, 2012, 26(4): 307-325. doi: 10.1080/08905436.2012.723606.
doi: 10.1080/08905436.2012.723606 |
| [54] |
GARAVAGLIA J, SCHNEIDER R DE C DE S, CAMARGO MENDES S D, WELKE J E, ZINI C A, CARAMÃO E B, VALENTE P. Evaluation of Zygosaccharomyces bailii BCV 08 as a co-starter in wine fermentation for the improvement of ethyl esters production. Microbiological Research, 2015, 173: 59-65.
doi: 10.1016/j.micres.2015.02.002 |
| [55] |
MARTÍNEZ-AVILA O, SÁNCHEZ A, FONT X, BARRENA R. Bioprocesses for 2-phenylethanol and 2-phenylethyl acetate production: current state and perspectives. Applied Microbiology and Biotechnology, 2018, 102(23): 9991-10004.
doi: 10.1007/s00253-018-9384-8 |
| [56] |
ZHANG B Q, TANG C, YANG D Q, LIU H, XUE J, DUAN C Q, YAN G L. Effects of three indigenous non-Saccharomyces yeasts and their pairwise combinations in co-fermentation with Saccharomyces cerevisiae on volatile compounds of Petit Manseng wines. Food Chemistry, 2022, 368: 130807.
doi: 10.1016/j.foodchem.2021.130807 |
| [57] |
张清安, 陈博宇. 葡萄酒中与风味相关4类含硫化合物的研究进展. 中国农业科学, 2020, 53(5): 1029-1045. doi: 10.3864/j.issn.0578-1752.2020.05.014.
doi: 10.3864/j.issn.0578-1752.2020.05.014 |
|
ZHANG Q A, CHEN B Y. Research progress of four sulfur compounds related to red wine flavor. Scientia Agricultura Sinica, 2020, 53(5): 1029-1045. doi: 10.3864/j.issn.0578-1752.2020.05.014. (in Chinese)
doi: 10.3864/j.issn.0578-1752.2020.05.014 |
|
| [58] |
ZHANG P Z, ZHANG R G, SIRISENA S, GAN R Y, FANG Z X. Beta-glucosidase activity of wine yeasts and its impacts on wine volatiles and phenolics: A mini-review. Food Microbiology, 2021, 100: 103859.
doi: 10.1016/j.fm.2021.103859 |
| [59] | 任学梅, 姚红红, 严幻汝, 祝霞, 杨学山. 高产糖苷酶非酿酒酵母菌株筛选、鉴定及其发酵过程中酶活性变化. 食品科学, 2022, 43(20): 198-206. |
| REN X M, YAO H H, YAN H R, ZHU X, YANG X S. Screening and identification of non-Saccharomyces yeast strains with high glycosidase production and changes in enzyme activities during their fermentation. Food Science, 2022, 43(20): 198-206. (in Chinese) | |
| [60] |
马延琴, 徐晓裕, 李甜, 李春燕, 蒋霞, 王斌, 史学伟. 酿酒葡萄表皮产酶非酿酒酵母的筛选及其产酶特性研究. 中国酿造, 2021, 40(12): 149-154. doi: 10.11882/j.issn.0254-5071.2021.12.027.
doi: 10.11882/j.issn.0254-5071.2021.12.027 |
|
MA Y Q, XU X Y, LI T, LI C Y, JIANG X, WANG B, SHI X W. Screening of non-Saccharomyces cerevisiae from wine grape epidermis and its enzyme-producing ability. China Brewing, 2021, 40(12): 149-154. doi: 10.11882/j.issn.0254-5071.2021.12.027. (in Chinese)
doi: 10.11882/j.issn.0254-5071.2021.12.027 |
|
| [61] |
ROMANO P, SUZZI G. Higher alcohol and acetoin production by Zygosaccharomyces wine yeasts. Journal of Applied Bacteriology, 1993, 75(6): 541-545.
doi: 10.1111/j.1365-2672.1993.tb01592.x |
| [62] |
PUERTAS B, JIMENEZ-HIERRO M J, CANTOS-VILLAR E, MARRUFO-CURTIDO A, CARBÚ M, CUEVAS F J, MORENO- ROJAS J M, GONZÁLEZ-RODRÍGUEZ V E, CANTORAL J M, RUIZ-MORENO M J. The influence of yeast on chemical composition and sensory properties of dry white wines. Food Chemistry, 2018, 253: 227-235.
doi: S0308-8146(18)30048-7 pmid: 29502826 |
| [63] |
ZHU L X, WANG G, AIHAITI A. Combined indigenous yeast strains produced local wine from over ripen Cabernet Sauvignon grape in Xinjiang. World Journal of Microbiology and Biotechnology, 2020, 36(8): 122.
doi: 10.1007/s11274-020-02831-4 |
| [64] |
DOMIZIO P, LIU Y, BISSON L F, BARILE D. Use of non- Saccharomyces wine yeasts as novel sources of mannoproteins in wine. Food Microbiology, 2014, 43: 5-15.
doi: 10.1016/j.fm.2014.04.005 |
| [65] |
DOMIZIO P, LENCIONI L, CALAMAI L, PORTARO L, BISSON L F. Evaluation of the yeast Schizosaccharomyces japonicus for use in wine production. American Journal of Enology and Viticulture, 2018, 69(3): 266-277.
doi: 10.5344/ajev.2018.18004 |
| [66] |
KIM D H, HONG Y A, PARK H D. Co-fermentation of grape must by Issatchenkiaorientalis and Saccharomyces cerevisiae reduces the malic acid content in wine. Biotechnology Letters, 2008, 30(9): 1633-1638.
doi: 10.1007/s10529-008-9726-1 |
| [67] |
HONG S K, LEE H J, PARK H J, HONG Y A, RHEE I K, LEE W H, CHOI S W, LEE O S, PARK H D. Degradation of malic acid in wine by immobilized Issatchenkia orientalis cells with oriental oak charcoal and alginate. Letters in Applied Microbiology, 2010, 50(5): 522-529. doi: 10.1111/j.1472-765X.2010.02833.x.
doi: 10.1111/j.1472-765X.2010.02833.x |
| [68] |
BELDA I, NAVASCUÉS E, MARQUINA D, SANTOS A, CALDERON F, BENITO S. Dynamic analysis of physiological properties of Torulaspora delbrueckii in wine fermentations and its incidence on wine quality. Applied Microbiology and Biotechnology, 2015, 99(4): 1911-1922.
doi: 10.1007/s00253-014-6197-2 |
| [69] |
白玉峰, 张文霞, 田亚楠, 张秀艳. 宁夏贺兰山东麓降L-苹果酸葡萄酒酵母的筛选. 中国酿造, 2021, 40(1): 49-54. doi: 10.11882/j.issn.0254-5071.2021.01.010.
doi: 10.11882/j.issn.0254-5071.2021.01.010 |
|
BAI Y F, ZHANG W X, TIAN Y N, ZHANG X Y. Screening of yeast strains with L-malic acid degradation ability from Eastern Foothills of Helan in Ningxia. China Brewing, 2021, 40(1): 49-54. doi: 10.11882/j.issn.0254-5071.2021.01.010. (in Chinese)
doi: 10.11882/j.issn.0254-5071.2021.01.010 |
|
| [70] |
MILHEIRO J, FILIPE-RIBEIRO L, VILELA A, COSME F, NUNES F M. 4-Ethylphenol, 4-ethylguaiacol and 4-ethylcatechol in red wines: Microbial formation, prevention, remediation and overview of analytical approaches. Critical Reviews in Food Science and Nutrition, 2019, 59(9): 1367-1391. doi: 10.1080/10408398.2017.1408563.
doi: 10.1080/10408398.2017.1408563 pmid: 29257912 |
| [71] |
FLEET G H. Wine yeasts for the future. FEMS Yeast Research, 2008, 8(7): 979-995. doi: 10.1111/j.1567-1364.2008.00427.x.
doi: 10.1111/j.1567-1364.2008.00427.x pmid: 18793201 |
| [72] |
KUCHEN B, MATURANO Y P, MESTRE M V, COMBINA M, TORO M E, VAZQUEZ F. Selection of native non-Saccharomyces yeasts with biocontrol activity against spoilage yeasts in order to produce healthy regional wines. Fermentation, 2019, 5: 60.
doi: 10.3390/fermentation5030060 |
| [73] |
COMITINI F, AGARBATI A, CANONICO L, GALLI E, CIANI M. Purification and characterization of WA18, a new mycocin produced by Wickerhamomyces anomalus active in wine against Brettanomyces bruxellensis spoilage yeasts. Microorganisms, 2020, 9: 56.
doi: 10.3390/microorganisms9010056 |
| [74] |
YAN W, GAO H, QIAN X J, JIANG Y J, ZHOU J, DONG W L, XIN F X, ZHANG W M, JIANG M. Biotechnological applications of the non-conventional yeast Meyerozyma guilliermondii. Biotechnology Advances, 2021, 46: 107674.
doi: 10.1016/j.biotechadv.2020.107674 |
| [75] |
BENITO S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Applied Microbiology and Biotechnology, 2018, 102(16): 6775-6790.
doi: 10.1007/s00253-018-9117-z |
| [76] |
BENITO S. The impacts of Schizosaccharomyces on winemaking. Applied Microbiology and Biotechnology, 2019, 103(11): 4291-4312.
doi: 10.1007/s00253-019-09827-7 |
| [77] | WANG B S, TAN F L, CHU R C, LI G Y, LI L B, YANG T Y, ZHANG M X. The effect of non-Saccharomyces yeasts on biogenic amines in wine. Trends in Food Science & Technology, 2021, 116: 1029-1040. |
| [78] |
GALLARDO-FERNÁNDEZ M, VALLS-FONAYET J, VALERO E, HORNEDO-ORTEGA R, RICHARD T, TRONCOSO A M, GARCIA-PARRILLA M C. Isotopic labelling-based analysis elucidates biosynthesis pathways in Saccharomyces cerevisiae for melatonin, serotonin and hydroxytyrosol formation. Food Chemistry, 2022, 374: 131742.
doi: 10.1016/j.foodchem.2021.131742 |
| [79] |
MORCILLO-PARRA M Á, GONZÁLEZ B, BELTRAN G, MAS A, TORIJA M J. Melatonin and glycolytic protein interactions are related to yeast fermentative capacity. Food Microbiology, 2020, 87: 103398.
doi: 10.1016/j.fm.2019.103398 |
| [80] |
WANG C X, WU C, QIU S Y. Yeast diversity investigation of Vitis davidii Föex during spontaneous fermentations using culture- dependent and high-throughput sequencing approaches. Food Research International, 2019, 126: 108582.
doi: 10.1016/j.foodres.2019.108582 |
| [81] | MASNEUF-POMAREDE I, BELY M, MARULLO P, ALBERTIN W. The genetics of non-conventional wine yeasts: Current knowledge and future challenges. Frontiers in Microbiology, 2016, 6: 1563. |
| [82] |
梁树英, 王春晓. 基于倍性的葡萄酒相关酵母酿造特性研究进展. 食品与发酵科技, 2022, 58(1): 121-130. doi: 10.3969/j.issn.1674-506X.2022.01-017.
doi: 10.3969/j.issn.1674-506X.2022.01-017 |
|
LIANG S Y, WANG C X. Research progress in wine-making characteristic of wine-related yeast based on ploidy. Sichuan Food and Fermentation, 2022, 58(1): 121-130. doi: 10.3969/j.issn.1674-506X.2022.01-017. (in Chinese)
doi: 10.3969/j.issn.1674-506X.2022.01-017 |
|
| [83] | ALBERTIN W, SETATI M E, MIOT-SERTIER C, MOSTERT T T, COLONNA-CECCALDI B, COULON J, GIRARD P, MOINE V, PILLET M, SALIN F, BELY M, DIVOL B, MASNEUF- POMAREDE I. Hanseniaspora uvarum from winemaking environments show spatial and temporal genetic clustering. Frontiers in Microbiology, 2016, 6: 1569. |
| [84] |
HRANILOVIC A, BELY M, MASNEUF-POMAREDE I, JIRANEK V, ALBERTIN W, FAIRHEAD C. The evolution of Lachancea thermotoleransis driven by geographical determination, anthropisation and flux between different ecosystems. PLoS ONE, 2017, 12(9): e0184652.
doi: 10.1371/journal.pone.0184652 |
| [85] |
WRENT P, RIVAS E M, PEINADO J M, DE SILÓNIZ M I. Development of an affordable typing method for Meyerozyma guilliermondii using microsatellite markers. International Journal of Food Microbiology, 2016, 217: 1-6.
doi: 10.1016/j.ijfoodmicro.2015.10.008 |
| [86] |
MASNEUF-POMAREDE I, JUQUIN E, MIOT-SERTIER C, RENAULT P, LAIZET Y, SALIN F, ALEXANDRE H, CAPOZZI V, COCOLIN L, COLONNA-CECCALDI B, ENGLEZOS V, GIRARD P, GONZALEZ B, LUCAS P, MAS A, NISIOTOU A, SIPICZKI M, SPANO G, TASSOU C, BELY M, ALBERTIN W. The yeast Starmerella bacillaris (synonym Candida zemplinina) shows high genetic diversity in winemaking environments. FEMS Yeast Research, 2015, 15(5): fov045. doi: 10.1093/femsyr/fov045.
doi: 10.1093/femsyr/fov045 |
| [87] |
ALBERTIN W, CHASSERIAUD L, COMTE G, PANFILI A, DELCAMP A, SALIN F, MARULLO P, BELY M, SCHACHERER J. Winemaking and bioprocesses strongly shaped the genetic diversity of the ubiquitous yeast Torulaspora delbrueckii. PLoS ONE, 2014, 9(4): e94246.
doi: 10.1371/journal.pone.0094246 |
| [88] |
田进, 吴成, 杨金仙, 王春晓. 葡萄酒有孢汉逊酵母属分子指纹图谱分析. 菌物学报, 2020, 39(4): 755-765. doi: 10.13346/j.mycosystema.190404.
doi: 10.13346/j.mycosystema.190404 |
|
TIAN J, WU C, YANG J X, WANG C X. The molecular fingerprinting analysis of Hanseniaspora in wine. Mycosystema, 2020, 39(4): 755-765. doi: 10.13346/j.mycosystema.190404. (in Chinese)
doi: 10.13346/j.mycosystema.190404 |
|
| [89] |
MENCHER A, MORALES P, CURIEL J A, GONZALEZ R, TRONCHONI J. Metschnikowia pulcherrima represses aerobic respiration in Saccharomyces cerevisiae suggesting a direct response to co-cultivation. Food Microbiology, 2021, 94: 103670.
doi: 10.1016/j.fm.2020.103670 |
| [90] |
LI Y Q, HU K, XU Y H, MEI W C, TAO Y S. Biomass suppression of Hanseniaspora uvarum by killer Saccharomyces cerevisiae highly increased fruity esters in mixed culture fermentation. LWT, 2020, 132: 109839.
doi: 10.1016/j.lwt.2020.109839 |
| [91] |
ROLLERO S, BLOEM A, BRAND J, ORTIZ-JULIEN A, CAMARASA C, DIVOL B. Nitrogen metabolism in three non-conventional wine yeast species: A tool to modulate wine aroma profiles. Food Microbiology, 2021, 94: 103650.
doi: 10.1016/j.fm.2020.103650 |
| [92] |
TANGULER H. Evaluation of Williopsissaturnus inoculum level on fermentation and flavor compounds of white wines made from emir (Vitis vinifera L.) grown in anatolia. Food Biotechnology, 2012, 26(4): 351-368.
doi: 10.1080/08905436.2012.724038 |
| [93] |
ROULLIER-GALL C, BORDET F, DAVID V, SCHMITT-KOPPLIN P, ALEXANDRE H. Yeast interaction on Chardonnay wine composition: Impact of strain and inoculation time. Food Chemistry, 2022, 374: 131732.
doi: 10.1016/j.foodchem.2021.131732 |
| [94] |
战吉宬, 曹梦竹, 游义琳, 黄卫东. 非酿酒酵母在葡萄酒酿造中的应用. 中国农业科学, 2020, 53(19): 4057-4069. doi: 10.3864/j.issn.0578-1752.2020.19.018.
doi: 10.3864/j.issn.0578-1752.2020.19.018 |
|
ZHAN J C, CAO M Z, YOU Y L, HUANG W D. Research advance on the application of non-Saccharomyces in winemaking. Scientia Agricultura Sinica, 2020, 53(19): 4057-4069. doi: 10.3864/j.issn.0578-1752.2020.19.018. (in Chinese)
doi: 10.3864/j.issn.0578-1752.2020.19.018 |
|
| [95] |
LIU H, LI X, DENG J Z, DAI L Y, LIU W, PAN B L, WANG C T, ZHANG D J, LI Z J. Molecular mechanism of the response of Zygosaccharomyces rouxii to D-fructose stress by the glutathione metabolism pathway. FEMS Yeast Research, 2020, 20(5): foaa034. doi: 10.1093/femsyr/foaa034.
doi: 10.1093/femsyr/foaa034 |
| [96] |
MBUYANE L L, BAUER F F, DIVOL B. The metabolism of lipids in yeasts and applications in oenology. Food Research International, 2021, 141: 110142.
doi: 10.1016/j.foodres.2021.110142 |
| [1] | 汪文娟,苏菁,陈深,杨健源,陈凯玲,冯爱卿,汪聪颖,封金奇,陈炳,朱小源. 广东省侵染美香占2号的稻瘟病菌致病性及无毒基因变异分析[J]. 中国农业科学, 2022, 55(7): 1346-1358. |
| [2] | 关若冰,李海超,苗雪霞. RNA生物农药的商业化现状及存在问题[J]. 中国农业科学, 2022, 55(15): 2949-2960. |
| [3] | 战吉宬,曹梦竹,游义琳,黄卫东. 非酿酒酵母在葡萄酒酿造中的应用[J]. 中国农业科学, 2020, 53(19): 4057-4069. |
| [4] | 徐云碧,杨泉女,郑洪建,许彦芬,桑志勤,郭子锋,彭海,张丛,蓝昊发,王蕴波,吴坤生,陶家军,张嘉楠. 靶向测序基因型检测(GBTS)技术及其应用[J]. 中国农业科学, 2020, 53(15): 2983-3004. |
| [5] | 穆心愿,赵霞,谷利敏,冀保毅,丁勇,张凤启,张君,齐建双,马智艳,夏来坤,唐保军. 秸秆还田量对不同基因型夏玉米产量及干物质转运的影响[J]. 中国农业科学, 2020, 53(1): 29-41. |
| [6] | 王倩倩,覃杰,马得草,陶永胜. 优选发酵毕赤酵母与酿酒酵母混合发酵增香酿造爱格丽干白葡萄酒[J]. 中国农业科学, 2018, 51(11): 2178-2192. |
| [7] | 石江鹏, 张春芬, 邓舒, 侯丽媛, 肖蓉, 李芙蓉, 董艳辉, 聂园军, 王亦学, 曹秋芬. 苹果纯合基因型新种质的性状鉴定与培养[J]. 中国农业科学, 2018, 51(10): 1960-1971. |
| [8] | 徐晴,许甫超,董静,董建辉,秦丹丹,鲁梦莹,李梅芳. 小麦氮素利用效率的基因型差异及相关特性分析[J]. 中国农业科学, 2017, 50(14): 2647-2657. |
| [9] | 温鑫,邓舒,张春芬,侯丽媛,石江鹏,聂园军,肖蓉,秦永军,曹秋芬. ‘嘎啦’苹果花药培养种质创新[J]. 中国农业科学, 2017, 50(14): 2793-2806. |
| [10] | 张徐非,候利娟,邱恒清,黄路生,郭源梅. 位置功能候选基因HMGA1、C6orf106和ENSSSCG00000023160与猪肢蹄结实度的关联性[J]. 中国农业科学, 2016, 49(20): 4030-4039. |
| [11] | 杨锦忠,梁淑敏,李娜娜,刘永花,郝建平. 玉米茎秆的支撑功能及其可塑性[J]. 中国农业科学, 2016, 49(1): 69-79. |
| [12] | 黄亿,李廷轩,张锡洲,戢林,吴沂珀. 氮高效利用基因型大麦氮素转移及氮形态组分特征[J]. 中国农业科学, 2015, 48(6): 1151-1161. |
| [13] | 徐云碧. 作物科学中的环境型鉴定(Envirotyping)及其应用[J]. 中国农业科学, 2015, 48(17): 3354-3371. |
| [14] | 曲志娜,刘红玉,王娟,赵思俊,李玉清,黄秀梅,盖文燕,王君玮. 青岛地区产ESBLs鸡源大肠杆菌耐药性调查与优势基因型分析[J]. 中国农业科学, 2015, 48(10): 2058-2066. |
| [15] | 戴海芳, 武辉, 阿曼古丽?买买提阿力, 王立红, 麦麦提?阿皮孜, 张巨松. 不同基因型棉花苗期耐盐性分析及其鉴定指标筛选[J]. 中国农业科学, 2014, 47(7): 1290-1300. |
|
||