













中国农业科学 ›› 2019, Vol. 52 ›› Issue (4): 639-650.doi: 10.3864/j.issn.0578-1752.2019.04.006
胡安华,祁静静,张庆雯,陈善春,邹修平,许兰珍,彭爱红,雷天刚,姚利晓,龙琴,何永睿( ),李强(
),李强( )
)
收稿日期:2018-10-13
接受日期:2018-11-26
出版日期:2019-02-16
发布日期:2019-02-27
通讯作者:
何永睿,李强
作者简介:胡安华,E-mail: 基金资助:
HU AnHua,QI JingJing,ZHANG QingWen,CHEN ShanChun,ZOU XiuPing,XU LanZhen,PENG AiHong,LEI TianGang,YAO LiXiao,LONG Qin,HE YongRui( ),LI Qiang(
),LI Qiang( )
)
Received:2018-10-13
Accepted:2018-11-26
Online:2019-02-16
Published:2019-02-27
Contact:
YongRui HE,Qiang LI
摘要:
【目的】克隆CsPGIP并分析其表达特性,转化柑橘得到超表达转基因株系,并进行柑橘溃疡病抗性评价,为柑橘溃疡病分子育种提供理论依据。【方法】从晚锦橙和四季橘中克隆柑橘CsPGIP,使用MEGA6进行多序列比对并构建系统发育树;采用在线软件BaCelLo和SignalP 4.0进行亚细胞定位和信号肽预测并用GFP瞬时表达确定CsPGIP在细胞内的定位;利用实时荧光定量PCR(qRT-PCR)比较接种溃疡病菌前后高感品种和高抗品种中柑橘CsPGIP的表达特性,分析溃疡病菌侵染与CsPGIP表达的相关性;农杆菌介导遗传转化晚锦橙,采用GUS染色初筛、PCR鉴定和qRT-PCR相结合的方法鉴定超表达转基因株系;观察转基因和野生型株系表型变化,分析其株高、叶片表型;离体针刺法对超表达转基因株系和野生型株系进行柑橘溃疡病抗性评价,统计病斑面积和病情指数,分析CsPGIP表达对柑橘抗、感溃疡病的影响。【结果】晚锦橙和四季橘CsPGIP均编码328个氨基酸,与已报道的柑橘中的PGIP同源性高达99.39%,都包含2个PGIP基因典型的LRR结构域(LRR_1和LRR_2);构建系统进化树发现甜橙中的CsPGIP与葡萄中的PGIP(GSVIVT01033370001)遗传距离最近,相似度达到62.97%,推测CsPGIP与葡萄中的PGIP具有类似的抗病效果。亚细胞定位和信号肽预测结果表明CsPGIP属于分泌蛋白,GFP洋葱瞬时表达证明柑橘CsPGIP定位在细胞膜和细胞壁,与预测结果一致。高感品种晚锦橙和高抗品种四季橘接种溃疡病菌后CsPGIP的表达特性不同,在高感品种中表达显著下调,而高抗品种中表达显著上调且维持在较高水平,推测CsPGIP与柑橘溃疡病的抗性相关。构建CsPGIP超表达载体并转化晚锦橙,通过PCR鉴定和qRT-PCR确定其中9个(OE1、OE3、OE4、OE5、OE6、OE9、OE10、OE12和OE14)为CsPGIP超表达阳性株系。通过对转基因株系的表型观察发现OE3、OE14株系表型与野生型株系相比差异明显,植株表现为较矮小,其中OE14出现叶片卷曲、增厚的表型变化。对CsPGIP超表达转基因株系(8个株系)进行离体抗溃疡病评价,结果显示超表达转基因株系可以使柑橘溃疡病病斑面积降至野生型的24.11%—83.88%,其中OE1株系的病斑面积最小;从病情指数来看,除OE3株系外,其余株系的病情指数均比野生型显著下降(为野生型的23.12%—75.49%),其中OE1下降最显著,综上结果可知超表达CsPGIP可以有效抑制柑橘溃疡病菌的生长。【结论】CsPGIP是柑橘响应溃疡病菌侵染的重要基因,可抑制或减轻柑橘溃疡病的发病程度,在柑橘抗溃疡病机理研究方面具有较大的应用价值,也可作为柑橘抗溃疡病分子育种的一个候选基因。
胡安华,祁静静,张庆雯,陈善春,邹修平,许兰珍,彭爱红,雷天刚,姚利晓,龙琴,何永睿,李强. 柑橘溃疡病相关基因CsPGIP的克隆与表达[J]. 中国农业科学, 2019, 52(4): 639-650.
HU AnHua,QI JingJing,ZHANG QingWen,CHEN ShanChun,ZOU XiuPing,XU LanZhen,PENG AiHong,LEI TianGang,YAO LiXiao,LONG Qin,HE YongRui,LI Qiang. Cloning and Expression Analysis of the Citrus Bacterial Canker-Related Gene CsPGIP in Citrus[J]. Scientia Agricultura Sinica, 2019, 52(4): 639-650.
表1
本研究使用的引物"
| 引物名称Primer name | 引物序列Primer sequence (5′-3′) | 酶切位点Enzyme site |
|---|---|---|
| OE-CsPGIP-f | CGGGATCCATGAGCAACACGTCACTGTTGTCT | BamHI |
| OE-CsPGIP-r | CGGAATTCTCACTTGCAGCTTTCGAGGGGCGC | EcoRI |
| SCL-CsPGIP-f | GGGGTACCATGAGCAACACGT CACTGTTGT | KpnI |
| SCL-CsPGIP-r | TCCCCCGGGCTTGCAGCTTTCG AGGGGCGCG | SmaI |
| qPCR-CsPGIP-f | AGAAGCTTGGCGCTCTTCAT | N/A |
| qPCR-CsPGIP-r | TCGCCTTCAAGCTTGTTCCT | N/A |
| qPCR-Actin-f | CATCCCTCAGCACCTTCC | N/A |
| qPCR-Actin-r | CCAACCTTAGCACTTCTCC | N/A |
| OE-f (35S) | AGTAAGGATCGAT CCCACAAAGT | N/A |
| OE-r (CsPGIP) | TTTTTGAAGAGTAGTGAAGCTGCA | N/A |
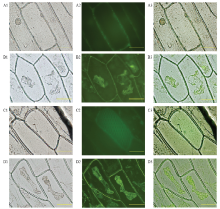
图3
CsPGIP的亚细胞定位 A1:明视野观察CsPGIP-GFP融合蛋白Image of CsPGIP-GFP under bright field;A2:暗视野观察CsPGIP-GFP融合蛋白Image of CsPGIP-GFP under dark field;A3:A1、A2视野叠加Overlap of A1 and A2;B1:明视野观察CsPGIP-GFP融合蛋白质壁分离Image of CsPGIP-GFP under bright field (plasmolysis);B2:暗视野观察CsPGIP-GFP融合蛋白质壁分离Image of CsPGIP-GFP under dark field (plasmolysis);B3:B1、B2视野叠加Overlap of B1 and B2;C1:明视野观察GFP表达Image of GFP under bright field;C2:暗视野观察GFP表达Image of GFP under dark field;C3:C1、C2视野叠加Overlap of C1 and C2;D1:明视野观察GFP质壁分离GFP of plasmolysis under bright field;D2:暗视野观察GFP质壁分离GFP of plasmolysis under dark field;D3:D1、D2视野叠加Overlap of D1 and D2;标尺Scale:100 μm"

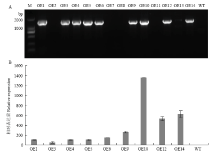
图5
转基因株系的鉴定及CsPGIP表达分析 A:CsPGIP转基因株系的PCR鉴定PCR amplification of CsPGIP in over-expression transgenic lines;B:转基因株系中CsPGIP的相对表达量检测The relative expression level of CsPGIP in over-expression transgenic lines。 M:分子量标准Marker;OE1—OE14:GUS初筛的转基因材料Lines verified from GUS staining;WT:野生型Wild-type;阳性株系特异扩增条带为1 530 bp PCR product size of positive lines is 1 530 bp"
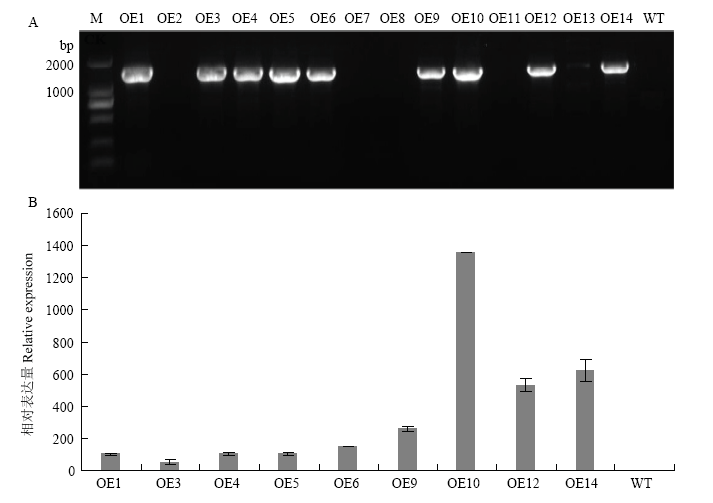
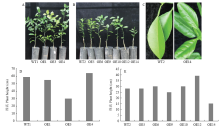
图6
转基因株系的表型分析 A:树龄1年的转基因株系(OE1、OE3、OE4)和野生型对照(WT1)植株The phenotype of 1-year-old transgenic lines (OE1, OE3, OE4) and the wild-type control (WT1);B:树龄6个月的转基因株系(OE5、OE6、OE9、OE10、OE12、OE14)和野生型对照(WT2)植株Phenotype of 6-month-old transgenic lines (OE5, OE6, OE9, OE10, OE12, OE14) and the wild-type control (WT2);C:野生型对照WT2和转基因株系OE14的叶片Leaves of WT2 and OE14;D:树龄1年的转基因株系(OE1、OE3、OE4)和野生型对照(WT1)的株高(测量方法:从嫁接口到顶梢的距离)Height of 1-year-old transgenic lines (OE1, OE3, OE4) and the wild-type control (measurement method: distance from the graft to the top tip);E:树龄6个月的转基因株系(OE5、OE6、OE9、OE10、OE12、OE14)和野生型对照(WT2)的株高Height of 6-month-old transgenic lines (OE5, OE6, OE9, OE10, OE12, OE14) and the wild-type control"
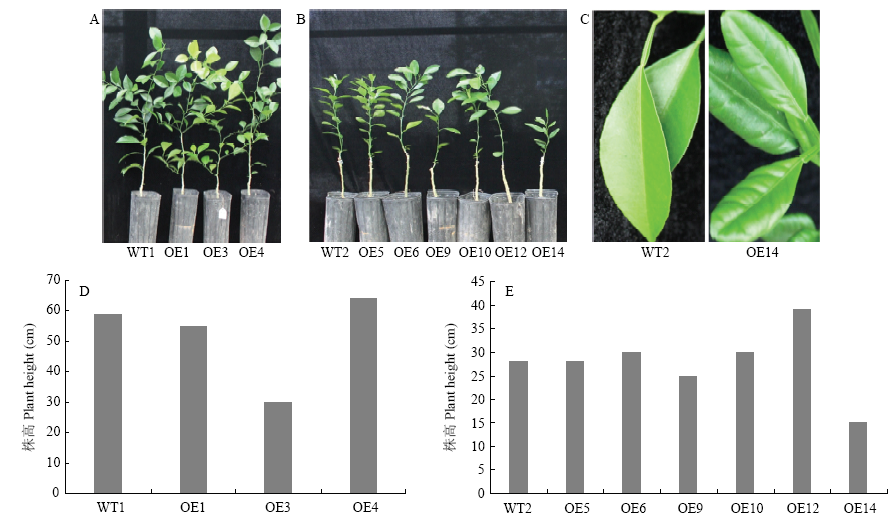
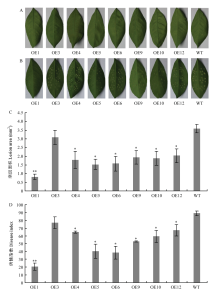
图7
转基因株系的抗性评价 A:接种LB培养基的转基因株系和野生型对照叶片Disease spots of transgenic lines and the wild-type inoculated with LB;B:接种溃疡病菌的转基因株系和野生型对照叶片Disease spots of transgenic lines and the wild-type inoculated with Xcc;C:接种溃疡病菌的转基因株系和野生型对照病斑面积Lesion area of transgenic lines and the wild-type inoculated with Xcc;D:接种溃疡病菌的转基因株系和野生型对照病情指数Disease index of transgenic lines and the wild-type inoculated with Xcc。WT:野生型对照Wild-type control;OE1—OE12:转基因株系Transgenic lines。*表示差异显著(P<0.05),**表示差异极显著(P<0.01)* represents significant difference (P<0.05), ** represents extremely significant difference (P<0.01)"

| [1] |
PITINO M, ARMSTRONG C M, DUAN Y P . Rapid screening for citrus canker resistance employing pathogen-associated molecular pattern-triggered immunity responses. Horticulture Research, 2015,2:15042.
doi: 10.1038/hortres.2015.42 pmid: 4595992 |
| [2] |
贾瑞瑞, 周鹏飞, 白晓晶, 陈善春, 许兰珍, 彭爱红, 雷天刚, 姚利晓, 陈敏, 何永睿, 李强 . 柑橘响应溃疡病菌转录因子CsBZIP40 的克隆及功能分析. 中国农业科学, 2017,50(13):2488-2497.
doi: 10.3864/j.issn.0578-1752.2017.13.008 |
|
JIA R R, ZHOU P F, BAI X J, CHEN S C, XU L Z, PENG A H, LEI T G, YAO L X, CHEN M, HE Y R, LI Q . Gene cloning and expression analysis of canker-related transcription factor CsBZIP40 in citrus. Scientia Agricultura Sinica, 2017,50(13):2488-2497. (in Chinese)
doi: 10.3864/j.issn.0578-1752.2017.13.008 |
|
| [3] | 杨枫, 陈传武, 范七君, 石春梅, 谢宗周, 郭大勇, 刘继红 . 温度和多胺对柑橘溃疡病发生的影响及作用机制. 中国农业科学, 2018,51(10):1899-1907. |
| YANG F, CHEN C W, FAN Q J, SHI C M, XIE Z Z, GUO D Y, LIU J H . Influence of temperature and polyamines on occurrence of citrus canker disease and underlying mechanisms. Scientia Agricultura Sinica, 2018,51(10):1899-1907. (in Chinese) | |
| [4] | 陈力, 王中康, 黄冠军, 曹月青, 夏玉先, 殷幼平 . 柑橘溃疡病生防菌株CQBS03的鉴定及其培养特性研究. 中国农业科学, 2008,41(8):2537-2545. |
| CHEN L, WANG Z K, HUANG G J, CAO Y Q, XIA Y X, YIN Y P . Evaluation of Bacillus subtilis strain CQBS03 against Xanthomonas axonopodis pv. citri. Scientia Agricultura Sinica, 2008,41(8):2537-2545. (in Chinese) | |
| [5] | 陈波, 罗庆华, 谭雅芹, 闫慧清 . 柑橘PGIP的B细胞抗原表位分析和原核表达. 现代食品科技, 2018,34(4):18-22. |
| CHEN B, LUO Q H, TAN Y Q, YAN H Q . B cell epitopes analysis and prokaryotic expression of PGIP in citrus. Modern Food Science and Technology, 2018,34(4):18-22. (in Chinese) | |
| [6] | FREIBERG A, MACHNER M P, PFELI W, SCHUBERT W D, HEINZ D W, SECKLER R . Folding and stability of the leucine-rich repeat domain of internal in B from Listeri monocytogenes. Journal of Molecular Biology, 2004,337(2):453-461. |
| [7] |
LEHMANN P . Structure and evolution of plant disease resistance genes. Journal of Applied Genetics, 2002,43(4):403-414.
pmid: 12441626 |
| [8] | FERRARI S, GALLETTI R, VAIRO D, GERVONE F, DE LORENZO G . Antisense expression of the Arabidopsis thaliana AtPGIP1 gene reduces polygalacturonase-inhibiting protein accumulation and enhances susceptibility to Botrytis cinerea. Molecular Plant-Microbe Interactions, 2006,19(8):931-936. |
| [9] | JOUBERT D A, KARS I, WAGEMAKERS L, BERGMANN C, KEMP G, VIVIER M A, VAN KAN J A . A polygalacturonase- inhibiting protein from grapevine reduces the symptoms of the endopolygalacturonase BcPG2 from Botrytis cinerea in Nicotiana benthamiana leaves without any evidence for in vitro interaction. Molecular Plant-Microbe Interactions, 2007,20(4):392-402. |
| [10] |
CHENG Q, CAO Y Z, PAN H X, WANG M X, HUANG M R . Isolation and characterization of two genes encoding polygalacturonase- inhibiting protein from Populus deltoides. Journal of Genetics and Genomics, 2008,35(10):631-638.
doi: 10.1016/S1673-8527(08)60084-3 pmid: 18937920 |
| [11] | HEGEDUS D D, LI R, BUCHWALDT L, PARKIN I, WHITWILL S, COUTU C, BEKKAOUI D, RIMMER S R . Brassica napus possesses an expanded set of polygalacturonase inhibitor protein genes that are differentially regulated in response to Sclerotinia sclerotiorum infection, wounding and defense hormone treatment. Planta, 2008,228(2):241-253. |
| [12] |
JANNI M, SELLA L, FAVARON F, BLECHL A E, DE LORENZO G, D’OVIDO R . The expression of a bean PGIP in transgenic wheat confers increased resistance to the fungal pathogenBipolaris sorokiniana. Molecular Plant-Microbe Interactions, 2008,21(2):171-177.
doi: 10.1094/MPMI-21-2-0171 pmid: 18184061 |
| [13] |
DI C X, LI M, LONG F, BAI M P, LIU Y J, ZHENG X L, XU S J, XIANG Y, SUN Z L, AN L Z . Molecular cloning, functional analysis and localization of a novel gene encoding polygalacturonase- inhibiting protein in Chorispora bungeana. Planta, 2009,231(1):169-178.
doi: 10.1007/s00425-009-1039-7 pmid: 19885675 |
| [14] | HWANG B H, BAE H, LIM H S, KIM K B, KIM S J, IM M H, PARK B S, KIM D S, KIM J . Overexpression of polygalacturonase- inhibiting protein 2 (PGIP2) of Chinese cabbage(Brassica rapa ssp. pekinensis) increased resistance to the bacterial pathogen Pectobacterium carotovorum ssp. carotovorum. Plant Cell, Tissue Organ Culture, 2010,103(3):293-305. |
| [15] |
D’OVIDIO R, RAIOLA A, CAPODICASA C, DEVOTO A, PONTIGGIA D, ROBERTI S, GALLETTI R, CONTI E, O’SULLIVAN D, DE LORENZO G . Characterization of the complex locus of bean encoding polygalacturonase-inhibiting proteins reveals subfunctionalization for defense against fungi and insects. Plant Physiology, 2004,135(4):2424-2435.
doi: 10.1104/pp.104.044644 |
| [16] |
LIU N N, ZHANG X Y, SUN Y, WANG P, LI X C, PEI Y K, LI F G, HOU Y X . Molecular evidence for the involvement of a polygalacturonase-inhibiting protein, GhPGIP1, in enhanced resistance to Verticillium and Fusarium wilts in cotton. Scientific Reports, 2017,7:39840.
doi: 10.1038/srep39840 pmid: 5228132 |
| [17] | JOUBERT D A, SLAUGHTER A R, KEMP C, BECKER J V, KROOSHOF C H, BERGMANN C, BENEN C, PRETORIUS I S, WIER M A . The polygalacturonase-inhibiting protein (VvPGIPl) reduces Botrytis cinerea susce in transgenic tobacco and differentially inhibits fungal polygalacturonases. Transgenic Research, 2006,15(6):687-702. |
| [18] |
BORRAS-HIDALGO O, CAPRARI C, HERNANDEZ-ESTEVEZ I, DE LORENZO G, CERVONE F . A gene for plant protection: expression of a bean polygalacturonase inhibitor in tobacco confers a strong resistance against Rhizoctonia solani and two oomycetes. Frontiers in Plant Science, 2012,3:268.
doi: 10.3389/fpls.2012.00268 pmid: 23264779 |
| [19] |
WANG A Y, WEI X N, RONG W, DANG L, DU L P, QI L, XU H J, SHAOY J, ZHANG Z Y . GmPGIP3 enhanced resistance to both take-all and common root rot diseases in transgenic wheat. Functional and Integrative Genomics, 2015,15(3):375-381.
doi: 10.1007/s10142-014-0428-6 pmid: 25487419 |
| [20] | MANFREDINI C, SICILIA F, FERRARI S, PONTIGGIA D, SALVI G, CAPRARI C, LORITO M, DE LORENZO G . Polygalacturonase- inhibiting protein 2 of Phaseolus vulgaris inhibits BcPGl, a polygalacturonase of Botrytis cinerea important for pathogenicity, and protects transgenic plants from infection. Physiological and Molecular Plant Pathology, 2005,67(2):108-115. |
| [21] |
PRABHU S A, WAGENKNECHT M, MELVIN P, KUMAR B S G, VEENA M, SHAILASREE S, MOERSCHBACHER B M, KINI K R . Immuno-affinity purification of PglPGIP1, a polygalacturonase inhibitor protein from pearl millet: studies on its inhibition of fungal polygalacturonases and role in resistance against the downy mildew pathogen. Molecular Biology Reports, 2015,42(6):1123-1138.
doi: 10.1007/s11033-015-3850-5 pmid: 255967221 |
| [22] |
PRABHU S A, KINI K R, RAJ S N, MOERSCHBACHER B M, SHETTY H S . Polygalacturonase-inhibitor proteins in pearl millet: possible involvement in resistance against downy mildew. Acta Biochimic et Biophysica Sinica, 2012,44(5):415-423.
doi: 10.1093/abbs/gms015 pmid: 22411686 |
| [23] | AGÜERO C B, URATSU S L, GREVE C, POWELL A T, LABAVITCH J M, MEREDITH C P, DANDEKAR A M . Evaluation of tolerance to Pierce’s disease and Botrytis in transgenic plants of Vitis vinifera L. expressing the pear PGIP gene. Molecular Plant Pathology, 2005,6(1):43-51. |
| [24] |
SCHACHT T, UNGER C, PICH A, WYDRA K . Endo- and exopolygalactuonases of Rslstonia solanacearum are inhibited by polygalactuonase-inhibiting protein (PGIP) activity in tomato stem extracts. Plant Physiology and Biochemistry, 2011,49(4):377-387.
doi: 10.1016/j.plaphy.2011.02.001 pmid: 21367611 |
| [25] |
WANG R, LU L, PAN X, HU Z, LING F, YAN Y, LIU Y, LIN Y . Functional analysis of OsPGIP1 in rice sheath blight resistance. Plant Molecular Biology, 2015,87(1/2):181-191.
doi: 10.1007/s11103-014-0269-7 pmid: 25488398 |
| [26] |
FENG C S, ZHANG X, WU T, YUAN B, DING X H, YAO F Y, CHU Z H . The polygalacturonase-inhibiting protein 4 (OsPGIP4), a potential component of the qBlsr5a locus, confers resistance to bacterial leaf streak in rice. Planta, 2016,243(5):1297-1308.
doi: 10.1007/s00425-016-2480-z pmid: 26945855 |
| [27] |
GOODSTEIN D M, SHU S, HOWSON R, NEUPANE R, HAYES R D, FAZO J, MITROS T, DIRKS W, HELLSTEN U, PUTNAM N, ROKHSAR D S . Phytozome: a comparative platform for green plant genomics. Nucleic Acids Research, 2012,40(Database issue):D1178-D1186.
doi: 10.1093/nar/gkr944 pmid: 22110026 |
| [28] |
TAMURA K, STECHER G, PETERSON D, FILIPSKI A, KUMAR S . MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution, 2013,30(12):2725-2729.
doi: 10.1093/molbev/mst197 pmid: 24132122 |
| [29] |
PIERLEONI A, MARTELLI P L, FARISELLI P, CASADIO R . BaCelLo: a balanced subcellular localization predictor. Bioinformatics, 2006,22(14):e408-e416.
doi: 10.1093/bioinformatics/btl222 |
| [30] |
PETERSEN T N, BRUNAK S, VON HEIJNE G, NIELSEN H . SignalP 4.0: discriminating signal peptides from transmembrane regions. Nature Methods, 2011,8(10):785-786.
doi: 10.1038/nmeth.1701 pmid: 21959131 |
| [31] | PENG A H, XU L Z, HE Y R, LEI T G, YAO L X, CHEN S C, ZOU X P . Efficient production of marker-free transgenic ‘Tarocco’ blood orange (Citrus sinensis Osbeck) with enhanced resistance to citrus canker using a Cre/loxP site-recombination system. Plant Cell, Tissue and Organ Culture, 2015,123(1):1-13. |
| [32] |
PENG A H, CHEN S C, LEI T G, XU L Z, HE Y R, WU L, ZOU X P . Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnology Journal, 2017,15(12):1509-1519.
doi: 10.1111/pbi.12733 pmid: 5698050 |
| [33] |
POWELL A L, VAN KAN J, TEN HAVE A, VISSER J, GREVE L C, BENNETT A B, LABAVITCH J M . Transgenic expression of pear PGIP in tomato limits fungal colonization. Molecular Plant-Microbe Interactions, 2000,13(9):942-950.
doi: 10.1094/MPMI.2000.13.9.942 pmid: 10975651 |
| [34] |
DE LORENZO G, D’OVIDIO R, CERVONE F . The role of polygalacturonase-inhibiting proteins (PGIPs) in defense against pathogenic fungi. Annual Review of Phytopathology, 2001,39(1):313-335.
doi: 10.1146/annurev.phyto.39.1.313 |
| [35] |
KOBE B, KAJAVA A V . The leucine-rich repeat as a protein recognition motif. Current Opinion in Structural Biology, 2001,11(6):725-732.
doi: 10.1016/S0959-440X(01)00266-4 pmid: 11751054 |
| [36] |
WANG X J, ZHU X P, TOOLEY P, ZHANG X G . Cloning and functional analysis of three genes encoding polygalacturonase- inhibiting proteins from Capsicum annuum and transgenic CaPGIP1 in tobacco in relation to increased resistance to two fungal pathogens. Plant Molecular Biology, 2013,81(4/5):379-400.
doi: 10.1007/s11103-013-0007-6 pmid: 23334855 |
| [37] | HU Y, ZHANG J L, JIA H G, SOSSO D, LI T, FROMMER W B, YANG B, WHITE F F, WANG N, JONES J B . Lateral organ boundaries 1 is a disease susceptibility gene for citrus bacterial canker disease. Proceedings of the National Academy of Sciences of the United States of America, 2014,111(4):E521-E529. |
| [1] | 肖桂华,文康,韩健,郝晨星,叶蓉春,朱亦赤,萧顺元,邓子牛,马先锋. 钙对枳生长发育及柑橘溃疡病抗性的影响[J]. 中国农业科学, 2022, 55(19): 3767-3778. |
| [2] | 冉宏标,赵丽玲,王会,柴志欣,王吉坤,王嘉博,武志娟,钟金城. LncFAM200B对牦牛肌内前体脂肪细胞脂质沉积的影响[J]. 中国农业科学, 2022, 55(13): 2654-2666. |
| [3] | 张婧芸,刘语诺,王兆昊,彭爱红,陈善春,何永睿. 转CiNPR4基因柑橘抗溃疡病的机制解析[J]. 中国农业科学, 2021, 54(18): 3871-3880. |
| [4] | 彭蕴,雷天刚,邹修平,张靖芸,张庆雯,姚家欢,何永睿,李强,陈善春. 柑橘溃疡病抗性SNP验证及其相关钙依赖性蛋白激酶基因诱导表达[J]. 中国农业科学, 2020, 53(9): 1820-1829. |
| [5] | 龙琴,杜美霞,龙俊宏,何永睿,邹修平,陈善春. 转录因子CsWRKY61对柑橘溃疡病抗性的影响[J]. 中国农业科学, 2020, 53(8): 1556-1571. |
| [6] | 秦秀娟,祁静静,窦万福,陈善春,何永睿,李强. 柑橘Rboh家族鉴定及其对激素和柑橘溃疡病的响应[J]. 中国农业科学, 2020, 53(20): 4189-4203. |
| [7] | 姚利晓,范海芳,张庆雯,何永睿,许兰珍,雷天刚,彭爱红,李强,邹修平,陈善春. 柑橘溃疡病抗性相关转录因子CitMYB20的功能[J]. 中国农业科学, 2020, 53(10): 1997-2008. |
| [8] | 邹修平,龙俊宏,彭爱红,陈敏,龙琴,陈善春. 超量表达CsGH3.6通过抑制生长素信号转导 增强柑橘溃疡病抗性[J]. 中国农业科学, 2019, 52(21): 3806-3818. |
| [9] | 窦万福,祁静静,胡安华,陈善春,彭爱红,许兰珍,雷天刚,姚利晓,何永睿,李强. GST pull-down联合LC-MS/MS筛选柑橘抗溃疡病转录因子CsBZIP40的互作蛋白[J]. 中国农业科学, 2019, 52(13): 2243-2255. |
| [10] | 贾瑞瑞,周鹏飞,白晓晶,陈善春,许兰珍,彭爱红,雷天刚,姚利晓,陈敏,何永睿,李强. 柑橘响应溃疡病菌转录因子CsBZIP40的克隆及功能分析[J]. 中国农业科学, 2017, 50(13): 2488-2497. |
| [11] | 丁林云, 张微, 王晋成, 田亮亮, 李妮娜, 郭琪, 杨淑明, 何曼林, 郭旺珍. 过量表达棉花GhSAP1提高转基因烟草的耐盐性[J]. 中国农业科学, 2014, 47(8): 1458-1470. |
| [12] | 殷幼平,袁训娥,李强,王中康 . 生防菌枯草芽孢杆菌CQBS03的绿色荧光蛋白基因标记及其在柑橘叶片上的定殖 [J]. 中国农业科学, 2010, 43(17): 3555-3563 . |
| [13] | 王中康,李泮志,袁青,于红,李蒙,殷幼平 . 柑橘溃疡病菌二硫键稳定Fv抗体基因的构建及表达产物的生物学活性分析[J]. 中国农业科学, 2009, 42(9): 3123-3130 . |
| [14] | . 抗PthA-NLS多肽单克隆抗体的制备及单链抗体基因的构建[J]. 中国农业科学, 2009, 42(3): 884-890 . |
| [15] | . 柑橘溃疡病生防菌株CQBS03的鉴定及其培养特性研究[J]. 中国农业科学, 2008, 41(8): 2537-2545 . |
|
||