













中国农业科学 ›› 2023, Vol. 56 ›› Issue (7): 1401-1416.doi: 10.3864/j.issn.0578-1752.2023.07.016
樊帅( ), 钟函, 杨中元, 何文瑞, 万博, 魏战勇, 韩世充(
), 钟函, 杨中元, 何文瑞, 万博, 魏战勇, 韩世充( ), 张改平(
), 张改平( )
)
收稿日期:2022-02-28
接受日期:2022-06-30
出版日期:2023-04-01
发布日期:2023-04-03
联系方式:
樊帅,Tel:15138698236;E-mail:fshuai9859@163.com。
基金资助:
FAN Shuai( ), ZHONG Han, YANG ZhongYuan, HE WenRui, WAN Bo, WEI ZhanYong, HAN ShiChong(
), ZHONG Han, YANG ZhongYuan, HE WenRui, WAN Bo, WEI ZhanYong, HAN ShiChong( ), ZHANG GaiPing(
), ZHANG GaiPing( )
)
Received:2022-02-28
Accepted:2022-06-30
Published:2023-04-01
Online:2023-04-03
摘要:
【背景】 非洲猪瘟(African swine fever,ASF)是由非洲猪瘟病毒(African swine fever virus,ASFV)感染引发的一种猪烈性传染病,是全球公认的养猪业“头号杀手”,至今尚无安全有效的疫苗和药物。病毒作为专性细胞内寄生物,必须通过“劫持”宿主翻译系统为病毒蛋白合成服务。其中翻译起始因子eIF2α作为翻译调控的核心节点,控制细胞应激反应和翻译重编程走向,对病毒毒力、嗜性、致病性及免疫逃逸等具有重要影响,eIF2α磷酸化调控无疑是病毒与宿主细胞竞争翻译资源的重要阵地之一。然而,关于ASFV编码蛋白与eIF2α磷酸化作用关系的认知极度匮乏。【目的】 探究非洲猪瘟病毒MGF110-5L-6L蛋白对宿主细胞翻译阻滞和促进应激颗粒形成的作用机制,为深入揭示非洲猪瘟病毒的致病机制研究提供科学依据。【方法】 在前期利用荧光素酶报告基因载体和绿色荧光报告载体,筛选发现外源表达MGF110-5L-6L极显著上调eIF2α磷酸化水平的基础上。选择猪肺泡巨噬细胞3D4/21和猪肾细胞PK-15作为研究用细胞系,利用质粒转染和特异性化学药物处理等方法,结合免疫印迹和激光共聚焦显微镜等检测技术,验证了MGF110-5L-6L蛋白与eIF2α磷酸化及其下游翻译效应之间的功能关系。随后,通过生物信息学网站预测、亚细胞定位和功能分析等,研究了MGF110-5L-6L蛋白与诱导细胞应激之间的相关性。【结果】 证实外源表达ASFV多基因家族蛋白MGF110-5L-6L能够显著增强细胞内eIF2α磷酸化水平及激活转录因子ATF4表达量,诱导综合应激反应的发生;还可诱导细胞发生内质网应激和未折叠蛋白反应,并通过活化PERK和PKR激酶来诱发eIF2α的磷酸化,进而导致细胞蛋白翻译阻滞和应激颗粒形成。进一步证实,MGF110-5L-6L蛋白含有两个高度保守的半胱氨酸基序,且主要定位于内质网,少量分布于高尔基体、线粒体和溶酶体,还可诱导高尔基体和过氧化物酶体的亚细胞定位及形态出现显著改变,提示其可能通过影响内质网腔中氧化还原稳态、蛋白分泌途径或相关膜性细胞器发生等创造应激条件。【结论】 报道了ASFV早期蛋白MGF110-5L-6L的结构、亚细胞定位及其潜在功能特征,揭示了MGF110-5L-6L蛋白与eIF2α磷酸化和宿主细胞蛋白翻译系统之间的功能关系,为深入认识ASFV的病原生物学与致病机制提供了新的科学参考。
樊帅, 钟函, 杨中元, 何文瑞, 万博, 魏战勇, 韩世充, 张改平. 非洲猪瘟病毒MGF110-5L-6L蛋白诱导宿主细胞翻译阻滞和应激颗粒形成的作用机制[J]. 中国农业科学, 2023, 56(7): 1401-1416.
FAN Shuai, ZHONG Han, YANG ZhongYuan, HE WenRui, WAN Bo, WEI ZhanYong, HAN ShiChong, ZHANG GaiPing. African Swine Fever Virus MGF110-5L-6L Induces Host Cell Translation Arrest and Stress Granule Formation by Activating the PERK/PKR-eIF2α Pathway[J]. Scientia Agricultura Sinica, 2023, 56(7): 1401-1416.
表1
蛋白质序列分析软件"
| 软件名称 Service name | 网站 Website | 功能 Function |
|---|---|---|
| ClustalX 2.1 | http://www.ebi.ac.uk/tools/clustalw2 | 多序列比对软件[ Multiple sequence alignment program |
| ESPript 3.0 | http://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi | 蛋白质多序列比对注释工具[ Generate a pretty PostScript output from aligned sequences |
| InterProScan | http://www.ebi.ac.uk/interpro/search/sequence/ | 蛋白质结构域和功能位点预测工具[ Predicting functional domains and important sites of proteins |
| SignalP 5.0 | https://services.healthtech.dtu.dk/service.php?SignalP-5.0 | 氨基酸序列信号肽预测工具[ Predict the presence of signal peptides and the location of their cleavage sites in proteins |
| TMHMM-2.0 | https://services.healthtech.dtu.dk/service.php?TMHMM-2.0 | 蛋白质跨膜α-螺旋预测工具[ Predict the topological structure of transmembrane proteins |
| NetNGlyc | https://services.healthtech.dtu.dk/service.php?NetNGlyc-1.0 | 蛋白质N-糖基化位点预测[ Predict N-Glycosylation sites in proteins |
| NetSurfP 2.0 | https://services.healthtech.dtu.dk/service.php?NetSurfP-2.0 | 预测氨基酸序列的表面可接近性和二级结构[ Predict secondary structure for each residue of the input sequences |
| I-TASSER | https://zhanggroup.org/I-TASSER/ | 蛋白质结构预测和基于结构的功能注释[ Protein structure prediction and structure-based function annotation |
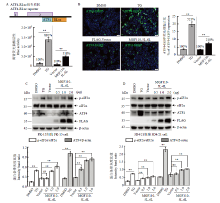
图1
外源表达MGF110-5L-6L蛋白显著上调eIF2α磷酸化水平 A:ATF4-RLuc报告质粒示意图(上)。将FLAG空载体或MGF110-5L-6L表达质粒与ATF4-RLuc共转染HEK 293T细胞,孵育24 h。在阳性对照组中,细胞经ATF4-RLuc转染18 h后,孵育DMSO或TG (1 μmol·L-1 ) 6 h,最后进行RLuc活性检测(下);B:将FLAG空载体或MGF110-5L-6L表达质粒与ATF4-EGFP共转染PK-15细胞,孵育24 h;或细胞转染ATF4-EGFP 18 h后,孵育DMSO或TG 6 h,并进行免疫荧光分析;C和D:将不同剂量的MGF110-5L-6L重组质粒或空载体转染PK-15细胞和3D4/21细胞,孵育24 h后,收集细胞裂解物,进行Western blot检测和灰度值分析。图中所示结果源自三次独立重复试验. **:P<0.01,n.s.:差异不显著"
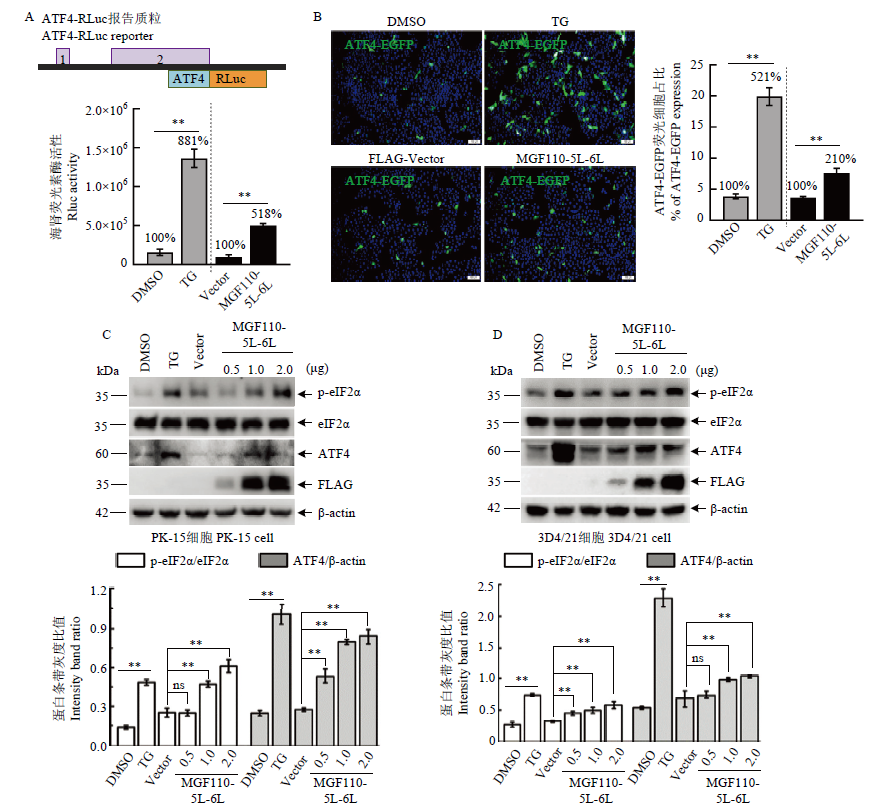
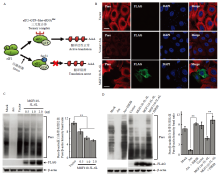
图2
外源表达MGF110-5L-6L蛋白诱导宿主蛋白翻译阻滞 A:eIF2α磷酸化调控宿主细胞翻译活性的模式图;B:将FLAG空载体或MGF110-5L-6L重组质粒转染PK-15细胞,孵育24 h后,利用嘌呤霉素(5 μg·mL-1)处理细胞30 min。随后细胞经固定和透膜处理后,分别孵育嘌呤霉素(红色)和FLAG(绿色)的抗体及相应二抗,细胞核用DAPI染色(蓝色),最后用激光共聚焦显微镜观察。Ars是蛋白翻译阻滞的诱导剂,利用0.5 mmol·L-1Ars预处理空载体转染细胞45 min后进行嘌呤霉素处理;C:将不同剂量的MGF110-5L-6L表达质粒或空载体转染PK-15细胞,孵育24 h后,加入5 μg·mL-1嘌呤霉素处理细胞30 min,随后收集细胞,并进行Western blot检测和灰度值分析。D:在经Ars处理和MGF110-5L-6L表达质粒转染的3D4/21细胞中加入0.5 μmol·L-1的ISRIB,收集细胞样品前,利用嘌呤霉素处理细胞以检测新生蛋白合成量。该结果源自三次独立重复试验. **:P<0.01"


图3
外源表达MGF110-5L-6L蛋白诱导应激颗粒SGs的形成 A:eIF2α磷酸化、应激颗粒和细胞蛋白翻译阻滞三者的关系图;B:分别将FLAG空载体或MGF110-5L-6L重组表达质粒转染PK-15细胞,孵育24 h,或伴随加入0.5 μmol·L-1 ISRIB孵育细胞;阳性对照组中,将FLAG空载体或DP71L重组质粒转染细胞,孵育24 h后,加入0.5 mmol·L-1 Ars处理细胞45 min。随后细胞经固定和透膜处理后,孵育G3BP1(绿色)和FLAG(红色)的抗体及相应二抗,细胞核用DAPI染色(蓝色),最后用激光共聚焦显微镜观察;C:用Image J分析每组SG阳性细胞所占比例。结果源自三次独立重复试验. **:P<0.01"
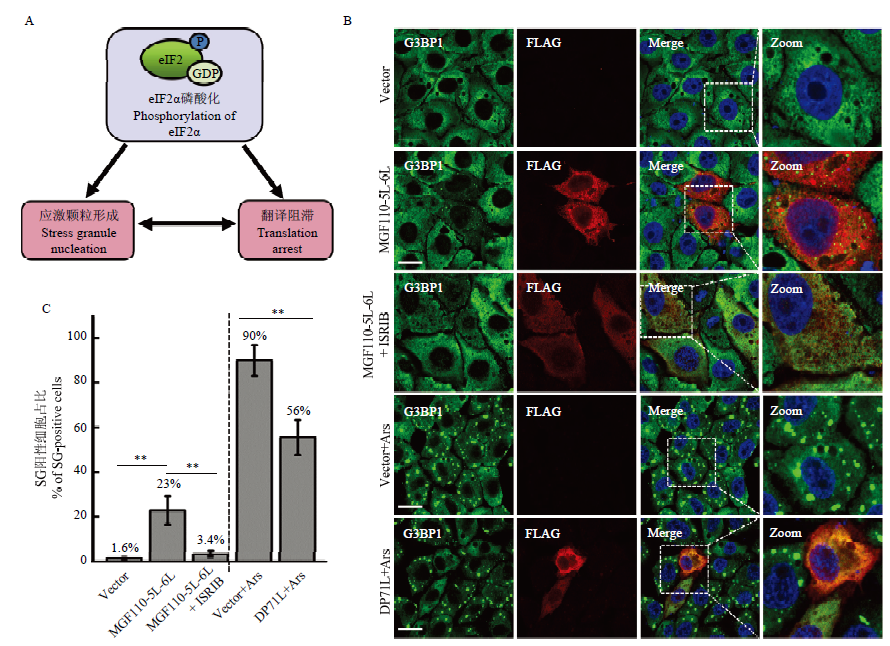
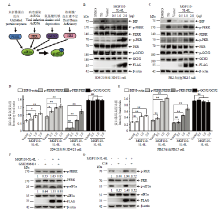
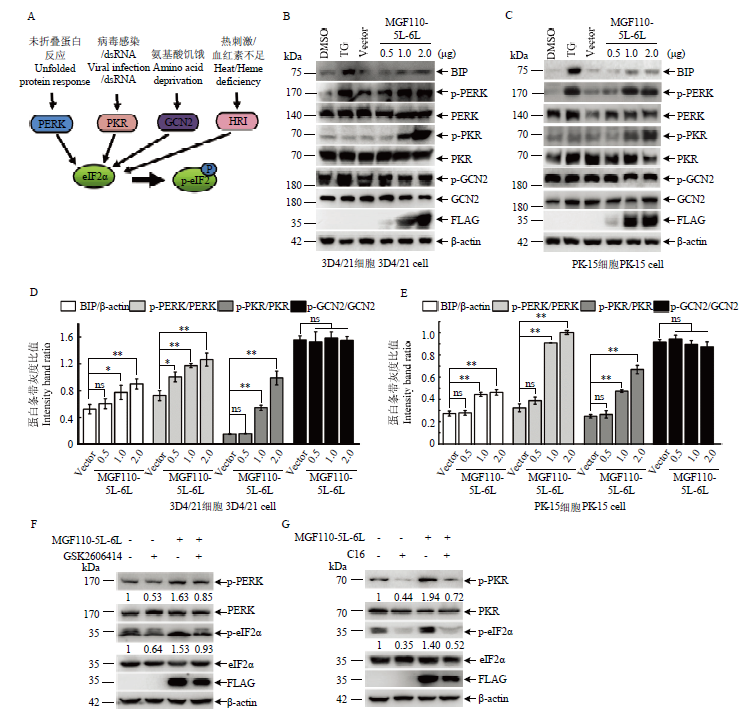
图6
外源表达MGF110-5L-6L蛋白通过活化PERK和PKR激酶诱导eIF2α磷酸化水平升高 A:PERK,PKR,GCN2,HRI 四种激酶诱导eIF2α磷酸化的模式图;B-E:将不同剂量的MGF110-5L-6L表达质粒或空载体转染3D4/21和PK-15细胞,孵育24 h后,收集细胞裂解物,进行Western blot检测和灰度值分析. 作为阳性对照,利用内质网应激诱导剂TG(1 μmol·L-1)孵育处理细胞6 h。F和G:分别将FLAG空载体或MGF110-5L-6L重组表达质粒转染3D4/21细胞,伴随10 μmol·L-1 GSK2606414或1 μmol·L-1 C16处理细胞,孵育24 h后,收集细胞进行Western blot检测;该结果源自三次独立重复试验. **, P<0.01"
| [1] |
doi: 10.1186/s40813-015-0013-y pmid: 28405426 |
| [2] |
doi: 10.1099/jgv.0.001049 |
| [3] |
doi: 10.3389/fimmu.2021.715582 |
| [4] |
doi: 10.1126/science.aaz1439 pmid: 31624094 |
| [5] |
doi: 10.1128/JVI.01293-18 |
| [6] |
doi: 10.1016/j.virusres.2019.197673 |
| [7] |
doi: 10.1038/nrmicro2655 pmid: 22002165 |
| [8] |
doi: 10.1146/annurev-virology-100114-055014 pmid: 27501262 |
| [9] |
doi: 10.1101/cshperspect.a033001 |
| [10] |
doi: 10.1101/cshperspect.a032870 |
| [11] |
doi: 10.1007/s00018-012-1252-6 |
| [12] |
doi: 10.1126/science.aat5314 |
| [13] |
doi: 10.15252/embr.201642195 |
| [14] |
doi: 10.1186/s12985-020-01362-6 pmid: 32703221 |
| [15] |
doi: 10.1016/0042-6822(81)90176-8 |
| [16] |
doi: 10.1371/journal.ppat.1000562 |
| [17] |
doi: 10.1128/JVI.00990-17 |
| [18] |
doi: 10.1128/JVI.00704-11 pmid: 21680527 |
| [19] |
doi: 10.1016/j.virusres.2012.10.025 pmid: 23154157 |
| [20] |
doi: 10.1128/JVI.00119-20 |
| [21] |
doi: 10.1128/jvi.01939-21 |
| [22] |
doi: 10.1073/pnas.2201288119 |
| [23] |
doi: 10.1073/pnas.0400541101 |
| [24] |
doi: 10.1093/bioinformatics/btm404 pmid: 17846036 |
| [25] |
doi: 10.1093/nar/gku316 |
| [26] |
doi: 10.1093/nar/gky1100 |
| [27] |
doi: 10.1038/s41587-019-0036-z pmid: 30778233 |
| [28] |
doi: 10.1006/jmbi.2000.4315 pmid: 11152613 |
| [29] |
|
| [30] |
doi: 10.1101/311209.doi:10.1101/311209 |
| [31] |
doi: 10.1016/j.crmeth.2021.100014 |
| [32] |
doi: 10.1016/S0166-0934(02)00085-X pmid: 12088830 |
| [33] |
doi: 10.1038/nmeth.1314 pmid: 19305406 |
| [34] |
doi: 10.1101/cshperspect. a032813 |
| [35] |
doi: 10.1128/JVI.01027-10 pmid: 20702639 |
| [36] |
doi: 10.1073/pnas.1501557112 |
| [37] |
doi: 10.1093/bib/bbaa380 |
| [38] |
doi: 10.1083/jcb.201610031 |
| [39] |
doi: 10.1128/jvi.78.7.3710-3721.2004 pmid: 15016891 |
| [40] |
doi: 10.16372/j.issn.1004-6364.2019.10.001 |
|
doi: 10.16372/j.issn.1004-6364.2019.10.001 |
|
| [41] |
doi: 10.1038/nri.2017.63 pmid: 28669985 |
| [42] |
doi: 10.1128/JVI.01906-16 |
| [43] |
doi: 10.3390/v13020286 |
| [44] |
doi: 10.1128/JVI.01872-20 |
| [45] |
doi: 10.1128/JVI.75.23.11755-11765.2001 pmid: 11689656 |
| [46] |
doi: 10.1128/JVI.00439-06 |
| [47] |
doi: 10.1099/vir.0.83343-0 |
| [48] |
doi: 10.1111/tbed.14242 pmid: 34314096 |
| [49] |
doi: 10.1128/JVI.03250-14 pmid: 25505073 |
| [50] |
doi: 10.1007/s12250-021-00350-6 pmid: 33689140 |
| [1] | 张冯禧,肖琦,朱家平,尹力鸿,赵霞玲,严明帅,徐晋花,温立斌,牛家强,何孔旺. 非洲猪瘟病毒P30蛋白单克隆抗体制备、鉴定及阻断ELISA方法的建立[J]. 中国农业科学, 2022, 55(16): 3256-3266. |
| [2] | 魏天,王成宇,王凤杰,李忠鹏,张芳毓,张守峰,扈荣良,吕礼良,王永志. 非洲猪瘟病毒p30蛋白单克隆抗体制备及线性抗原表位定位[J]. 中国农业科学, 2022, 55(15): 3062-3070. |
| [3] | 张静远,缪发明,陈腾,李敏,扈荣良. 非洲猪瘟实时荧光RPA诊断方法建立及应用[J]. 中国农业科学, 2022, 55(1): 197-207. |
| [4] | 王涛,韩玉,潘力,王冰,孙茂文,王翌,罗玉子,仇华吉,孙元. 针对非洲猪瘟病毒MGF360-13L基因的TaqMan荧光定量PCR的建立[J]. 中国农业科学, 2021, 54(5): 1073-1080. |
|
||