













中国农业科学 ›› 2023, Vol. 56 ›› Issue (7): 1311-1321.doi: 10.3864/j.issn.0578-1752.2023.07.009
杨岭1( ), 田晓丽2, 桂连友1, 王福莲1, 张国辉1(
), 田晓丽2, 桂连友1, 王福莲1, 张国辉1( )
)
收稿日期:2022-12-10
接受日期:2023-01-17
出版日期:2023-04-01
发布日期:2023-04-03
联系方式:
杨岭,E-mail:202071666@yangtzeu.edu.cn。
基金资助:
YANG Ling1( ), TIAN XiaoLi2, GUI LianYou1, WANG FuLian1, ZHANG GuoHui1(
), TIAN XiaoLi2, GUI LianYou1, WANG FuLian1, ZHANG GuoHui1( )
)
Received:2022-12-10
Accepted:2023-01-17
Published:2023-04-01
Online:2023-04-03
摘要:
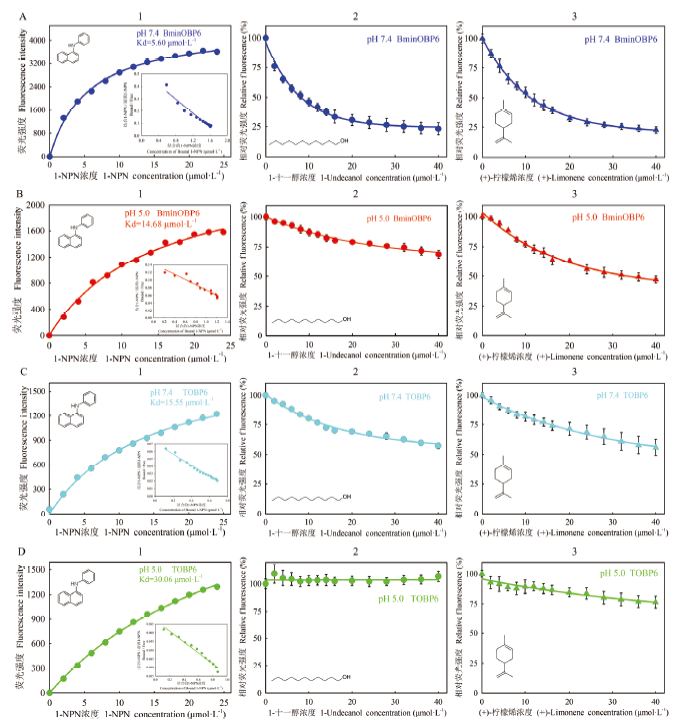
【目的】 通过构建柑橘大实蝇(Bactrocera minax)BminOBP6的C末端截短突变体(TOBP6),比较野生型BminOBP6和突变体TOBP6对气味配体的结合能力,检测在不同pH条件下二者对气味配体结合能力的差异,为揭示BminOBP6与配体的互作机制提供参考。【方法】 通过在线工具SWISS MODEL对BminOBP6进行同源建模,根据构建的3D模型确定将被切除的BminOBP6第6个α螺旋(α6)之后的C末端序列;设计特异性引物扩增BminOBP6的C末端截短突变体(TOBP6)的编码序列,即TOBP6;构建重组表达载体pET32a/TOBP6,将此重组载体与实验室保存的重组载体pET32a/BminOBP6分别转入原核细胞BL21(DE3)中,异源表达野生型BminOBP6及其突变体TOBP6;以1-NPN为荧光探针进行荧光竞争结合试验,检测BminOBP6和TOBP6在两种pH环境(pH 7.4和pH 5.0)下对1-十一醇和(+)-柠檬烯的结合能力。【结果】 序列比对结果显示,BminOBP6与致倦库蚊(Culex quinquefasciatus)CquiOBP1的序列具有很高的相似性,为62.6%,远高于30%,因此选择以CquiOBP1的3D结构作为BminOBP6同源建模模板。模型显示BminOBP6的α6之后是由8个氨基酸残基(D117—P124)组成的C末端序列,即将被切除的C末端序列。在此基础上设计引物克隆获得了编码TOBP6的cDNA序列,并构建了其重组表达载体pET32a/TOBP6,该重组载体和pET-32a/BminOBP6重组载体分别转入大肠杆菌细胞中进行了异源表达。荧光竞争结合试验结果显示,在pH为7.4时,BminOBP6与1-十一醇和(+)-柠檬烯具有很强的结合能力,Ki值分别为6.89和9.50 μmol·L-1。当pH降至5.0时,BminOBP6却丧失了对1-十一醇的结合能力,同时与(+)-柠檬烯的结合能力也大幅减弱,Ki值从9.50 μmol·L-1升至31.26 μmol·L-1;而不论在何种pH条件下,TOBP6均丧失了对这两种气味配体的结合能力。【结论】 pH在柑橘大实蝇 BminOBP6与其配体结合和释放过程中发挥了重要作用,并且BminOBP6的C末端在配体的结合中起着关键作用。
杨岭, 田晓丽, 桂连友, 王福莲, 张国辉. 柑橘大实蝇气味结合蛋白BminOBP6与其气味配体的互作机制[J]. 中国农业科学, 2023, 56(7): 1311-1321.
YANG Ling, TIAN XiaoLi, GUI LianYou, WANG FuLian, ZHANG GuoHui. Interaction Mechanisms Between Bactrocera minax Odorant-Binding Protein BminOBP6 and Its Ligands[J]. Scientia Agricultura Sinica, 2023, 56(7): 1311-1321.
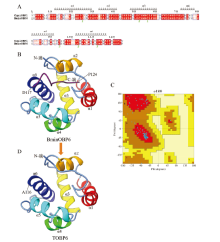
图1
BminOBP6同源建模构建的三维结构 A:BminOBP6与模板蛋白CquiOBP1氨基酸序列同源性比对Sequence alignment between BminOBP6 and CquiOBP1;B:BminOBP6三维结构3D structure of BminOBP6;C:BminOBP6 Pro-CHECK质量评估结果The result of Pro-CHECK quality assessment of 3D structure of BminOBP6;D:BminOBP6的C末端截短突变体(TOBP6)三维结构3D structure of BminOBP6 C terminus-truncated mutant (TOBP6)"
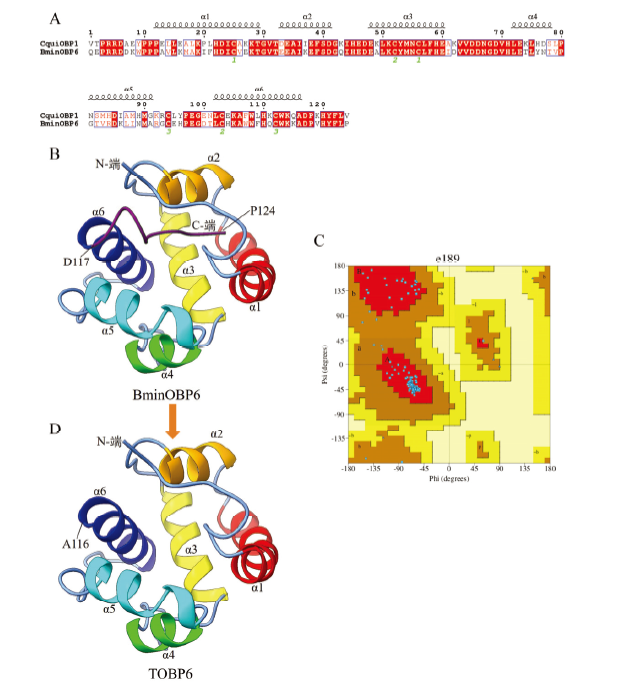
图3
pET-32a/BminOBP6与pET-32a/TOBP6的表达和纯化产物的SDS-PAGE分析 A:1:未经诱导的pET-32a/BminOBP6表达产物Expression products of pET-32a/BminOBP6 without induction;2:经诱导的pET-32a/BminOBP6表达产物Expression products of pET-32a/BminOBP6 with induction;3:BminOBP6上清The supernatant of BminOBP6;4:BminOBP6包涵体The inclusion body of BminOBP6;5:BminOBP6带有His-Tag BminOBP6 with His-Tag;6:BminOBP6去除His-Tag BminOBP6 without His-Tag;M1:宝生物蛋白分子量标准TaKaRa protein molecular weight marker;M2:赛默飞预染蛋白分子量标准Thermo scientific pageruler prestained protein ladder。B:1:未经诱导的pET-32a/TOBP6表达产物Expression products of pET-32a/TOBP6 without induction;2:经诱导的pET-32a/TOBP6表达产物Expression products of pET-32a/TOBP6 with induction;3:TOBP6上清The supernatant of TOBP6;4:TOBP6包涵体The inclusion body of TOBP6;5:TOBP6带有His-Tag TOBP6 with His-Tag;6:TOBP6去除His-Tag TOBP6 without His-Tag;M2:赛默飞预染蛋白分子量标准Thermo scientific pageruler prestained protein ladder"
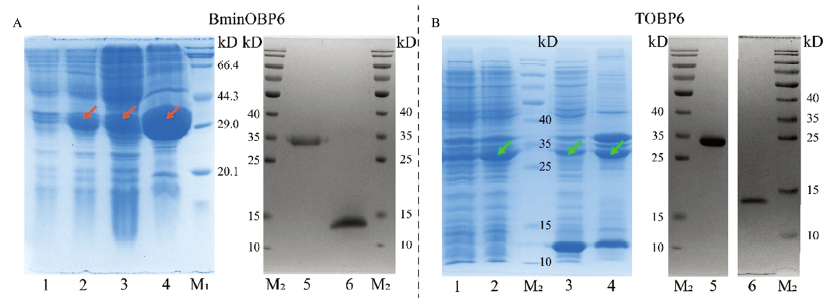
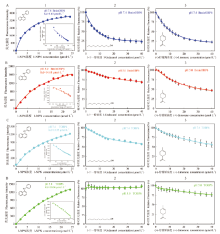
图4
BminOBP6和TOBP6的荧光竞争结合特性 A:pH 7.4条件下的BminOBP6 BminOBP6 at pH 7.4;B:pH 5.0条件下的BminOBP6 BminOBP6 at pH 5.0;C:pH 7.4条件下的TOBP6 TOBP6 at pH 7.4;D:pH 5.0条件下的TOBP6 TOBP6 at pH 5.0。1:与1-NPN的结合曲线及Scatchard方程Binding curves and Scatchard plots of protein and 1-NPN;2:与1-十一醇的竞争结合曲线Binding curves of protein to 1-undecanol;3:与(+)-柠檬烯的竞争结合曲线Binding curves of protein to (+)-limonene"
| [1] |
陈世骧, 谢蕴贞. 关于桔大实蝇的学名及其种征. 昆虫学报, 1955, 5(1): 123-126.
|
|
|
|
| [2] |
龚碧涯, 刘慧, 肖伏莲, 向敏, 刘娟, 杨水芝, 李先信, 段科平, 熊先福. 粘虫胶、食物诱芯和悬挂位置对诱杀球诱杀柑橘大实蝇的影响. 应用昆虫学报, 2019, 56(4): 840-845.
|
|
|
|
| [3] |
doi: 10.4236/ae.2018.62005 |
| [4] |
李可,
|
|
|
|
| [5] |
doi: 10.1002/(ISSN)1521-1878 |
| [6] |
|
| [7] |
doi: 10.1146/annurev-ento-120811-153635 pmid: 23020622 |
| [8] |
pmid: 16332206 |
| [9] |
doi: 10.1007/s10886-008-9485-4 pmid: 18535862 |
| [10] |
doi: 10.1016/j.ibmb.2009.03.007 pmid: 19364529 |
| [11] |
吴帆, 张莉, 邱一蕾, 李红亮. 昆虫嗅觉结合蛋白研究进展. 昆虫学报, 2021, 64(4): 523-535.
|
|
|
|
| [12] |
白鹏华, 王冰, 张仙红, 王桂荣. 昆虫气味受体的研究方法与进展. 昆虫学报, 2022, 65(3): 364-385.
|
|
|
|
| [13] |
|
| [14] |
doi: 10.1111/brv.2018.93.issue-1 |
| [15] |
doi: 10.1126/science.1121249 |
| [16] |
doi: 10.1371/journal.pone.0009471 |
| [17] |
doi: S0960-9822(16)31198-8 pmid: 27916525 |
| [18] |
doi: 10.1016/j.jmb.2009.04.015 |
| [19] |
doi: 10.1016/j.jmb.2011.10.005 |
| [20] |
doi: 10.1016/S1074-5521(00)00078-8 |
| [21] |
doi: 10.1016/j.bbrc.2005.07.176 |
| [22] |
doi: 10.1016/j.chembiol.2009.01.005 |
| [23] |
doi: 10.1016/j.jmb.2004.01.009 |
| [24] |
doi: 10.1016/j.jmb.2005.10.015 |
| [25] |
doi: 10.1021/bi9020132 |
| [26] |
doi: 10.1016/j.bbrc.2005.10.191 |
| [27] |
doi: 10.1371/journal.pone.0008006 |
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
doi: 10.3390/ijms23031190 |
| [32] |
doi: 10.1093/jee/toab199 |
| [33] |
pmid: 13990617 |
| [34] |
doi: 10.1002/(ISSN)1522-2683 |
| [35] |
doi: 10.1093/nar/gkg520 pmid: 12824332 |
| [36] |
doi: 10.1016/j.jinsphys.2012.04.010 |
| [37] |
doi: 10.3389/fevo.2020.00063 |
| [38] |
doi: 10.1006/bbrc.2000.2158 |
| [39] |
|
| [40] |
doi: 10.1038/354408a0 |
| [41] |
doi: 10.1038/s41598-020-60242-9 |
| [42] |
doi: 10.1002/pro.v22.1 |
| [43] |
doi: 10.1021/bi901145a |
| [1] | 赵慧婷,彭竹,姜玉锁,赵淑果,黄丽,杜亚丽,郭丽娜. 中华蜜蜂气味结合蛋白AcerOBP7的表达及结合特性[J]. 中国农业科学, 2022, 55(3): 613-624. |
| [2] | 徐翔,解屹,宋丽云,申莉莉,李莹,王勇,刘明宏,刘东阳,王小彦,赵存孝,王凤龙,杨金广. 高效靶向降解烟草花叶病毒核酸的dsRNA筛选与大量制备[J]. 中国农业科学, 2021, 54(6): 1143-1153. |
| [3] | 刘孝贺,仇贵生,佟兆国,张怀江,闫文涛,岳强,孙丽娜. 桃小食心虫化学感受蛋白CSP16配体结合特性[J]. 中国农业科学, 2021, 54(5): 945-958. |
| [4] | 秦健辉,李金桥,赵旭,李克斌,曹雅忠,尹姣. 铜绿丽金龟气味结合蛋白AcorOBP11的表达纯化及功能分析[J]. 中国农业科学, 2021, 54(14): 3017-3028. |
| [5] | 解昆仑,刘莉铭,刘美,彭斌,吴会杰,古勤生. 小西葫芦黄花叶病毒dsRNA的原核表达及其对ZYMV的防治效果[J]. 中国农业科学, 2020, 53(8): 1583-1593. |
| [6] | 王佳,王攀,樊欢,刘映红. 柑橘大实蝇滞育型与非滞育型蛹的代谢谱比较[J]. 中国农业科学, 2019, 52(6): 1021-1031. |
| [7] | 毕可然,李银,韩凯凯,赵冬敏,刘青涛,刘宇卓,黄欣梅,杨婧. 鸭寡腺苷酸合成酶样蛋白原核表达及多克隆抗体制备[J]. 中国农业科学, 2019, 52(23): 4429-4436. |
| [8] | 李玲,谭瑶,周晓榕,庞保平. 沙葱萤叶甲气味结合蛋白GdauOBP20的基因克隆、 原核表达及其结合特性[J]. 中国农业科学, 2019, 52(20): 3705-3712. |
| [9] | 李都, 牛长缨, 李峰奇, 罗晨. 斑翅果蝇气味结合蛋白OBP56h与小分子化合物的结合特征[J]. 中国农业科学, 2019, 52(15): 2616-2623. |
| [10] | 王会,柴志欣,朱江江,钟金城,张成福,信金伟. 牦牛Linc24063的克隆鉴定及其与miRNAs 表达水平的相关性分析[J]. 中国农业科学, 2019, 52(14): 2538-2547. |
| [11] | 易敏,吕青,刘柯柯,王礼君,吴玉娇,周泽扬,龙梦娴. 家蚕微孢子虫极管蛋白2(NbPTP2)的表达、纯化和定位特征[J]. 中国农业科学, 2019, 52(10): 1830-1838. |
| [12] | 张奎,李重阳,苏晶晶,谈娟,徐曼,崔红娟. 家蚕整合素β2的表达、纯化及其免疫功能[J]. 中国农业科学, 2019, 52(1): 181-190. |
| [13] | 赵洁,任苏伟,刘宁,艾新宇,马纪,刘小宁. 棉铃虫磷脂酰乙醇胺结合蛋白的克隆及表达分析[J]. 中国农业科学, 2018, 51(8): 1493-1503. |
| [14] | 陈东凯,张林雅,邢振龙,雷仲仁. 美洲斑潜蝇气味结合蛋白OBP13的鉴定与功能[J]. 中国农业科学, 2018, 51(5): 893-904. |
| [15] | 植爽,任艳红,唐星,徐凤翔,王传宏,赵爱春,王茜龄. 桑树谷氨酸脱氢酶基因MaGDHs的克隆及表达分析[J]. 中国农业科学, 2018, 51(4): 758-769. |
|
||