0 引言
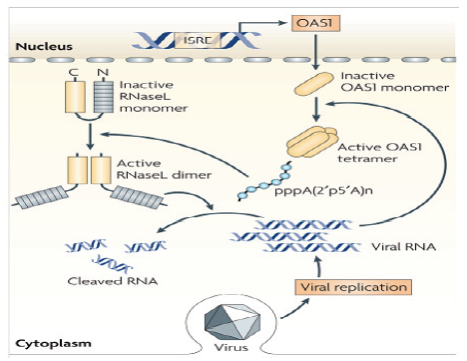
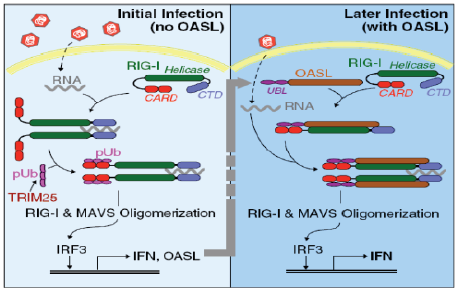
【研究意义】养鸭业是我国的特色产业和农村经济发展的支柱产业之一,随着养殖规模的扩大和养殖密度的提高,各种病害频繁发生,病毒病是危害养鸭业健康发展的重要疫病之一。鸭寡腺苷酸合成酶样蛋白(duck oligoadenylate synthetase-like protein,DuOASL)是寡腺苷酸合成酶家族蛋白的一种,已有研究表明OAS家族蛋白是一种具有广谱的抗病毒活性蛋白,当人被病毒感染后,人OASL蛋白快速通过C端泛素化区与视黄酸诱导基因蛋白I(retinoic acid inducible gene-I,RIG-I)的CARDs结构域结合,进而激活RIG-I信号通路,增强机体细胞检测病毒RNA的能力,继而激活机体免疫系统来感知病毒并且抑制病毒复制[1]。因此,基于笔者前期的研究结果,通过原核表达和多克隆抗体制备技术获得带有GST标签的原核表达蛋白和多克隆抗体对深入探讨鸭寡腺苷酸合成酶样蛋白抑制病毒复制分子机制奠定坚实物质基础。【前人研究进展】在禽类中,日本学者首次证实鸡体内OAS 家族蛋白为OASL蛋白,该蛋白可以抑制黄病毒属的西尼罗河病毒复制[2];最近,TAG-EL-DIN-HASSAN等[3,4]进一步证实,鸡OASL蛋白不仅具有重要的抗病毒特性,而且该蛋白的抗病毒特性不依赖于2—5寡腺苷酸合成酶。我国学者杨超等也报道鹅体内仅有OASL蛋白,过表达鹅OASL能抑制新城疫病毒在鹅胚成纤维细胞中的复制[5]。韩凯凯等基于鸭卵泡组织差异蛋白组学分析发现鸭OASL蛋白在鸭坦布苏病毒感染组和未感染组表达存在差异,在病毒感染组中表达明显增强[6]。前期研究首次获得了鸭OASL基因全长,蛋白结构分析表明,鸭OASL蛋白N端具有寡腺苷酸合成酶样结构域,C端具有泛素化样结构域,并且寡腺苷酸合成酶样结构域中具有保守的寡腺苷酸合成酶活性位点,预测可能具有寡腺苷酸合成酶的特性[7]。HASSAN 等[8] 2018年对鸭、鹅和鸵鸟的OAS基因进行了功能分析,结果证实鸭、鹅和鸡的OASL一样,既具有寡腺苷酸合成酶特性,又具有不依赖于寡腺苷酸合成酶的抗黄病毒特性;而鸵鸟的OASL只具有抗黄病毒特性,不具有OAS1蛋白的寡腺苷酸合成酶特性。在哺乳动物OAS家族蛋白抗病毒研究中,一些学者证实OAS蛋白可以激活受体内切核糖核酸酶L(Endoribonuclease L,RNase L),二者结合后可以通过OAS/RNaseL途径实现抗病毒的作用,进而降解感染细胞中的RNA,有效阻止RNA病毒的复制(图1)[9],该信号通路在宿主控制黄病毒、呼肠孤病毒、脑心肌炎病毒和流感病毒的先天性免疫反应中起重要作用[10,11,12]。此外,鸭OASL蛋白C端泛素化样结构域与人OASL蛋白相似性高达96.2%[13],而人OASL蛋白C端泛素化结构域是RIG-I信号通路激活的关键区域(图2)[14]。2015 年,IBSEN 等[15]证实人OASL 蛋白C端泛素化结构域可以取代K63连接的多泛素化链与RIG-I蛋白的CARDs结构域结合,N端寡腺苷酸合成酶样结构域具有一个结合dsRNA的凹槽,二者协同作用不仅可以检测病毒RNA,还可以激活RIG-I信号通路,对病毒RNA具有监测和抑制作用。之后,ALCORN 等[16]也证实哺乳动物OASL蛋白具有抵抗流感病毒NS1 蛋白介导的免疫逃逸潜力,同时,不像RIG-I蛋白,过表达的OASL蛋白不会激活干扰素因子,进而不会产生对宿主有毒副作用的其他产物。DHAR等[17]也发现哺乳动物OASL蛋白能够克服呼吸道合胞体病毒NS1介导的免疫逃逸,实现对病毒复制的抑制。BI等[13]通过鸭OASL蛋白过表达和干扰表达在细胞学水平证实其可以抑制鸭坦布苏病毒的复制。HU等 [18] 通过对数据库中已有动物OAS家族蛋白序列及结构进行系统分析后发现,即使没有dsRNA刺激,古老的OAS蛋白也具有2—5和3—5寡腺苷酸合成酶特性。然而受进化压力影响,高等后生动物OAS蛋白与dsRNA的亲和能力增强并且仅具有2—5寡腺苷酸合成酶特性,研究结果为后生动物体内OASs的功能研究提供新的思路。WANG等 [19] 通过分子克隆技术获得了猪OASL基因全长,序列分析表明,该基因C端不具有泛素化区域。通过RNAi沉默和过表达表明,猪OASL基因N端31—60氨基酸序列是抗病毒复制的关键区域。YAO等[20] 通过分子克隆技术获得了树鼩OAS家族全部基因,分析表明树鼩OAS家族有OAS1、OAS2、OASL1和OASL2四个基因,OAS1和OAS2具有抗病毒特性,而OASL1和OASL2不具有抗病毒特性。【本研究切入点】 尽管从分子生物学角度掌握了鸭OASL蛋白的结构特点并发现其有抗病毒复制的特性,但其抗病毒分子机制仍然是一片空白,而且在其它禽类研究中也无相关报道。【拟解决的关键问题】本研究基于前期获得的OASL基因全长,通过RT-PCR扩增、载体构建和蛋白纯化等方法获得带有GST标签的OASL融合蛋白,并通过免疫方法获得鸭OASL蛋白多克隆抗体,为进一步研究鸭OASL蛋白抗病毒复制机理奠定基础。
图1
图2
1 材料与方法
1.1 试验材料
动物总RNA提取试剂盒购自Omega公司,樱桃谷鸭幼鸭、含有GST标签的原核表达载体pGEX-4t-1和大肠杆菌BL21(PLYSS)TM为江苏省农业科学院兽医研究所家禽重大疫病防控项目组保存提供;限制性内切酶BamH I和Xho I购自宝生物工程(大连)有限公司;DNA聚合酶购自日本Toyobo公司;琼脂糖凝胶回收试剂盒、小量提取质粒试剂盒、无缝克隆试剂盒、鼠源GST标签抗体、辣根过氧化物酶标记山羊抗小鼠IgG和ECL发光液均购自北京全式金生物技术有限公司;新西兰白兔购自江苏省农科院种兔场;其他试剂均为进口分装或国产分析纯。所有试验均在江苏省农业科学院兽医研究所家禽重大疫病防控研究室完成。
1.2 PCR扩增
2017年4月,根据已经获得的鸭OASL基因ORF序列设计原核表达PCR扩增引物pGEX-OAS-F:5′-G GATCTGGTTCCGCGTGGATCCATGGAGCTGTGGA ACGTGTC-3′和pGEX-OAS-R: 5′-CAGTCACGATGC GGCCGCTCGAGTCAGGAGGACGGGCAGCCG-3′,以江苏省农业科学院兽医研究所动物房养殖的樱桃谷鸭幼鸭脾脏cDNA为模板进行PCR扩增,PCR 反应体系50 μL:33 μL 灭菌水,5 μL 10×Buffer,5 μL dNTPs(2 mmol·L-1),3 μL Mg2+,1 μL 脾脏cDNA,1 μL正反向引物,1 μL KOD-Plus-neo。PCR条件:94 ℃ 2min;98 ℃ 10 s、68 ℃ 1min(30个循环);68℃ 7min。扩增产物经1%琼脂糖凝胶电泳,然后利用胶回收试剂盒回收目的条带。
1.3 重组表达质粒构建与鉴定
将目的条带和带有GST标签的pGEX-4t-1载体分别用BamH I和Xho I双酶切,酶切产物用无缝克隆试剂盒37℃反应45 min,转化到BL21(PLYSS)TM感受态细胞中,构建重组表达质粒pGEX-4t-1-OASL。经菌落PCR鉴定后,挑选阳性重组表达质粒送上海英俊公司测序。
1.4 OASL融合蛋白诱导表达与蛋白分析
鉴定过的重组表达菌株接种于5 mL LB培养基(含50 μg·mL-1氨苄霉素)中,37℃培养过夜,次日取50 μL接种于5 mL的LB培养基(含50 μg·mL-1氨苄霉素)中,37℃震荡培养至菌液浓度OD600达0.4—0.7左右时,取出1 mL作对照,剩余部分加入IPTG至终浓度为0.5 mmol·L-1进行诱导,诱导4 h后收菌液,诱导后的菌液分别经12 000 r/min离心5 min,弃上清,沉淀用菌体裂解液垂悬,超声波破碎30 min,取50 μL破碎混合液后剩余液体12 000 r/min离心5 min,上清液转移到新离心管中,沉淀用PBS重悬,诱导前混合液、诱导后破碎混合液、上清和沉淀分别与4×蛋白电泳上样缓冲液混匀,沸水浴5 min后经SDS-PAGE和Western-bloting检测蛋白表达情况。
1.5 OASL融合蛋白的纯化
鉴定过的重组表达菌株接种于5 mL LB培养基(含50 μg·mL-1氨苄霉素)中,37℃培养过夜,次日取50 μL接种于5 mL的LB培养基(含50 μg·mL-1氨苄霉素)中,37℃震荡培养至菌液浓度OD600达0.4—0.7左右时,取出1 mL作对照,剩余部分加入IPTG至终浓度为0.5 mmol·L-1进行诱导,诱导4 h后收菌液,超声破碎,将上清用0.45 μm滤膜过滤后加入GST亲和层析柱中,洗脱液洗脱后将收集的蛋白溶液加入透析袋中,对未纯化的蛋白、透出液及洗脱液进行SDS-PAGE分析,检测目的蛋白的纯化效果,并用BCA法测定纯化蛋白的浓度。
1.6 OASL融合蛋白多克隆抗体的制备
用纯化和测定的OASL 重组蛋白先后3次皮下多点注射免疫新西兰大白兔。第一次免疫采用弗氏完全佐剂和蛋白样品按1﹕1混合,超声破碎仪乳化后免疫,蛋白免疫量为每只400 μg。二周以后二免,纯化的蛋白与不完全弗氏佐剂等量乳化后免疫,间隔一周后加强免疫,最后一次免疫后的14 d,心脏采血收集血清。
1.7 OASL融合蛋白多克隆抗体的纯化与鉴定
将蛋白OASL与琼脂糖介质偶联制备成抗原亲和纯化层析柱,将所得抗血清与PBS等量混合后缓慢上样,待抗体结合后用甘氨酸洗脱缓冲液洗脱,即得到所需纯化抗体,立即在PBS溶液中进行4℃透析过夜,隔日通过ELISA检测纯化抗体的效价,利用BCA 法测定纯化蛋白的浓度,通过SDS-PAGE电泳观察纯化抗体的纯度。
2 结果
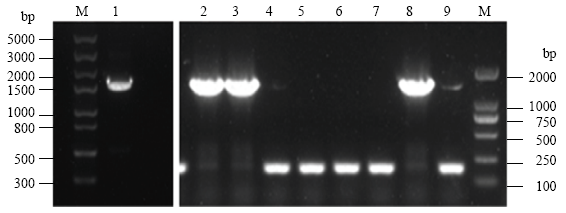
2.1 鸭全长OASL基因扩增和重组表达质粒pGEX- 4t-1-OASL的鉴定
图3
图3
鸭OASL基因RT-PCR和菌落PCR扩增结果。
M:左Left:Trans5K DNA marker,右Right:Trans2K DNA marker; 1:RT-PCR; 2-9:菌落PCR Colony PCR
Fig. 3
Agrose gel electrophoresis patterns of duck OASL gene by RT-PCR and colony PCR
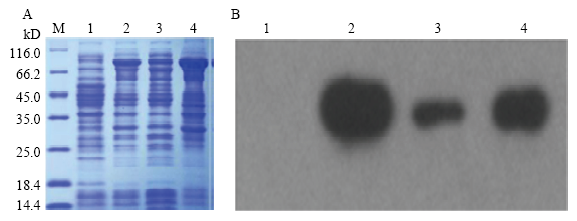
2.2 融合蛋白诱导表达与蛋白分析
图4
图4
融合蛋白SDS-PAGE和Western-blotting检测
M:蛋白marker;1:IPTG诱导前;2:IPTG诱导后全菌;3:IPTG诱导后沉淀;4:IPTG诱导后上清
Fig. 4
The result of SDS-PAGE and Western-blotting from recombinant protein
M: Protein marker; 1: Before IPTG induction; 2: All bacteria induced by IPTG; 3: Precipitation induced by IPTG; 4: Supernatant induced by IPTG
2.3 融合蛋白的纯化
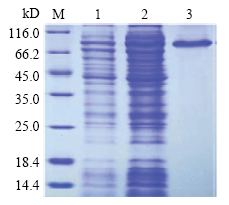
鉴定过的重组表达菌株经过扩大体积培养后,将所获菌体进行超声破碎,离心后取上清液,用GST标签亲和层析柱对上清液中的可溶性目的蛋白进行富集,多次洗涤除去多余杂蛋白,最后用GST Elution- Buffer(20 mmol·L-1 Tris-HCl,50 mmol·L-1 GSH,0.15 mol·L-1 NaCl,pH8.0)以1 mL·min-1流速洗脱目的蛋白,收集流出液,上述收集的蛋白溶液加入透析袋中,使用20 mmol·L-1 Tris-HCl,0.10 mol·L-1 NaCl,pH8.0进行透析过夜,经12%的SDS-PAGE 胶电泳检测,在83 kD附近获得一条明显条带,其分子量大小与理论值相符,同时洗脱液中蛋白所含杂带较少,说明获得纯度较高的目的蛋白(图5)。将目的蛋白用BCA法测定其浓度达0.5 mg·mL-1,作为免疫动物的抗原。
图5
图5
融合蛋白纯化后SDS-PAGE检测
M:蛋白marker; 1:未纯化重组蛋白;2:流出液;3:洗脱液
Fig. 5
The result of SDS-PAGE from the purified recombinant protein
M: Protein marker; 1: Unpurified recombinant protein; 2: Effluent; 3: Eluent
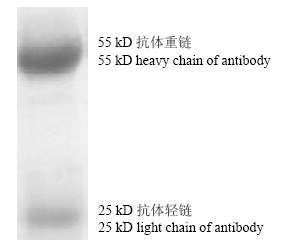
2.4 多克隆抗体的鉴定
OASL蛋白通过抗原亲和纯化层析柱纯化后,经SDS-PAGE检测可见55 kD左右有一明亮单一的目的条带,与预期结果一致(图6)。结果表明,纯化后的多克隆血清纯度较高。ELISA 方法检测结果显示,重组OASL蛋白浓度为5 μg·mL-1时,OASL家兔多克隆血清效价为1:512 000。
图6
3 讨论
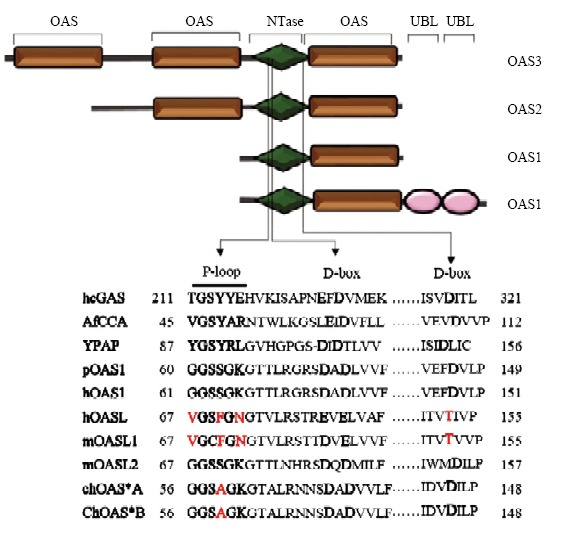
寡腺苷酸合成酶(oligoadenylate synthase,OAS)是干扰素诱导机体产生的一种重要抗病毒蛋白,在宿主先天性免疫、信号转导等过程中发挥重要的作用。该酶最早在干扰素处理后的人体细胞中发现,而后在哺乳动物、鸟类和低等生物如海绵动物体内也陆续被发现和报道 [21,22]。OAS家族蛋白包括OAS1、OAS2、OAS3和OASL等4个成员,OAS1、OAS2、OAS3和OASL均有核苷酸转移酶结构域(nucleotidyl transferase,NTase),但各有1、2、3和1个OAS 结构域,OASL比起其他3个成员在C 端多了两个泛素样结构域(ubiquitin-like,UBL)(图7)[23],该泛素化区可以与RIG-I蛋白结合,进而激活RIG-I信号通路,实现对病毒增殖的抑制,该结果已经在人、鼠和猪OASL蛋白研究中证实[24,25,26,27]。OAS2和OAS3蛋白仅分布在哺乳动物中,OAS1和OASL蛋白广泛分布于各种动物体内[28]。人的OASL基因和鸡的OASL基因 有很高的同源性,但是鸡的OASL蛋白却具有很强的酶活性[29]。前期的研究表明,鸭OASL蛋白与其他已报到的禽类OASL蛋白具有很高的同源性,N端的NTase结构域和寡腺苷酸合成酶样结构域都有保守的P-loop、D-box、LIRL、YALELLT、RPVILDPADP区及关键的赖氨酸和精氨酸,这些序列保守区对寡腺苷酸合成酶活性的发挥起着决定性作用[13]。在细胞学水平,通过体外试验证实鸭OASL蛋白能够抑制鸭坦布苏病毒的复制,但在体内,由于缺少相应蛋白的特异性抗体,所以,当鸭被病毒感染后,无法从蛋白质水平对DuOASL进行监测和分析。本试验基于前期获得鸭脾脏cDNA文库,利用RT-PCR方法将鸭OASL基因成功克隆到高效表达载体pGEX-4t-1中进行表达,通过改变诱导剂IPTG 的用量和诱导时间找到表达量最高的条件,对OASL蛋白进行分离纯化,免疫家兔,获得抗OASL的多克隆血清,经间接ELISA 鉴定,多抗血清抗体效价较高,同时,原核表达的OASL融合蛋白具有GST标签抗体,为今后从DuOASL蛋白水平监测鸭被病毒感染状况和从蛋白水平筛选与DuOASL蛋白互作的病毒蛋白奠定物质基础。
图7
4 结论
通过原核表达技术获得了带有GST标签的鸭寡腺苷酸合成酶样蛋白融合蛋白,蛋白纯度是0.5 mg·mL-1。通过免疫学方法获得了鸭寡腺苷酸合成酶样蛋白多克隆抗体,抗体效价为1:51 200。
参考文献
Antiviral activity of human OASL protein is mediated by enhancing signaling of the RIG-I RNA sensor
DOI:10.1016/j.immuni.2014.05.007
URL
[本文引用: 1]

Virus infection is sensed in the cytoplasm by retinoic acid-inducible gene I (RIG-I, also known as DDX58), which requires RNA and polyubiquitin binding to induce type I interferon (IFN) and activate cellular innate immunity. We show that the human IFN-inducible oligoadenylate synthetases-like (OASL) protein has antiviral activity and mediates RIG-I activation by mimicking polyubiquitin. Loss of OASL expression reduced RIG-I signaling and enhanced virus replication in human cells. Conversely, OASL expression suppressed replication of a number of viruses in a RIG-I-dependent manner and enhanced RIG-I-mediated IFN induction. OASL interacted and colocalized with RIG-I, and through its C-terminal ubiquitin-like domain specifically enhanced RIG-I signaling. Bone-marrow-derived macrophages from mice deficient for Oasl2 showed that among the two mouse orthologs of human OASL, Oasl2 is functionally similar to human OASL. Our findings show a mechanism by which human OASL contributes to host antiviral responses by enhancing RIG-I activation.
2′,5′-oligoadenylate synthetase gene in chicken: gene structure, distribution of alleles and their expression
DOI:10.1016/s0167-4781(00)00174-3
URL
PMID:11121584
[本文引用: 1]

We have cloned the gene for chicken 2',5'-oligoadenylate synthetase (ChOAS) by the method of polymerase chain reaction with use of ChOAS cDNA sequence. The ChOAS gene is composed of five introns and six exons containing all of the sequence of the ChOAS cDNA from the start to the stop codon. The first five exons of ChOAS gene which encode the OAS catalytic domain have a similar structure to HuOAS1 gene including the exon-intron boundaries. However, the length of introns of ChOAS gene is only 1/7 of those of HuOAS1 gene. The sixth exon of the ChOAS gene encodes the ubiquitin-like (UbL) domain of two consecutive sequence (UbL1 and UbL2) homologous to ubiquitin. ChOAS encoded in a single copy gene has at least two alleles, OAS(*)A and OAS(*)B. The differences between these two alleles are in the sixth exon of the gene; a 96-nucleotide sequence in the UbL1 portion of OAS(*)A is deleted from OAS(*)B. No OAS(*)B gene was detected in nine lines of chickens tested other than Leghorns. Almost the same levels of ChOAS-A and -B proteins induced physiologically in erythrocytes were detected in infant chickens (2-week-old), but in grown-up chickens (6-month-old) the level of erythrocyte OAS-B was markedly reduced in most of B/B chickens. Thus, the UbL domain of ChOAS is responsible for the maintenance of the OAS level in the tissue.
Analysis of the relationship between enzymatic and antiviral activities of the chicken oligoadenylate synthetase-like
DOI:10.1089/jir.2016.0012
URL
PMID:27849431
[本文引用: 1]

The oligoadenylate synthetase (OAS) is well known as an antiviral factor against the flavivirus infection in mammals. It is known that the oligoadenylate synthetase-like (ChOAS-L) gene is only present in the chicken genome. It has been shown in the previous report that the ChOAS-L possesses enzymatic activity to convert ATP into 2'-5'-linked oligoadenylates and antiviral activity against West Nile virus (WNV) replicon. Therefore, this study aimed to investigate the relationship between enzymatic and antiviral activities of ChOAS-L. Eight mutated ChOAS-L proteins were generated using either the site-directed mutagenesis or standard polymerase chain reaction protocol. The wild-type and mutated proteins were ectopically expressed in 293FT cells to analyze the enzymatic activity and in BHK-21 and BALB/3T3 cells to analyze the antiviral activity using WNV replicon. The results revealed that all mutated proteins showed no enzymatic activity except for ChOAS-L-AΔUbL2. However, all mutated proteins showed antiviral activity to inhibit the replication of the WNV replicon except for ChOAS-L-AΔUbL1/UbL2, which showed a partial inhibition compared to the wild-type ChOAS-L-A or other mutated proteins. These results suggest that the ChOAS-L expresses the antiflavivirus activity in a manner independent of enzymatic activity. Our results propose reconsideration of the mechanism of antiviral activity against the flavivirus replication of ChOAS-L.
The chicken 2′-5′ oligoadenylate synthetase A inhibits the replication of West Nile virus
West Nile virus (WNV) is a pathogen to cause West Nile encephalitis when the infection occurs in the brain. Previous studies in mice identified the 2'-5' oligoadenylate synthetase 1b (Oas1b) gene as a determining factor for resistance to WNV infection. In addition, it has been suggested that human OAS1 and OASL are associated with the resistance to the WNV infection. WNV is maintained in nature through a complex life cycle involving wild birds and mosquitoes. Birds are not only susceptible to the WNV, but also act as reservoir hosts, thus participating in the spread of the disease. It has previously been reported that chicken OASL possesses the oligoadenylate synthetase activity. However, until now the antiviral activity of chicken OASL has not been determined. In this study, we investigated the putative antiviral activity of chicken OASL by ectopic expression of this enzyme in mammalian cells and then infecting these cells with WNV replicon. We demonstrate that chicken OASL has an antiviral activity against the WNV. This is the first report to show that chicken OASL is associated with the resistance to the WNV infection.
Identification of 2′-5′-oligoadenylate synthetase-like gene in goose: gene structure, expression patterns, and antiviral activity against Newcastle Disease Virus
DOI:10.1016/j.meegid.2019.104139
URL
PMID:31841700
[本文引用: 1]

Bone marrow stromal cell antigen 2 (BST2) is an interferon induced host restriction factor for HIV-1 that blocks the release of nascent virions from infected T cells. We aimed to characterize BST2 gene variants in HIV-1 positive individuals in Indian cohort and study the association of these variants with disease progression in long term non progressors (LTNPs) and progressors. Archived samples of 32 LTNPs, 17 progressors, and 78 controls were screened for BST2 gene polymorphisms using Sanger's sequencing method. Frequency distribution, survival analysis and bioinformatics tools were used to study the association of BST2 variants with disease progression. Eighteen variants of BST2 gene were observed in Indian cohort. Intronic SNP rs919267T/C (OR = 4.489 [0.8494-27.03], p = .04157) and exonic SNP rs13485C/G (OR = 3.887 [0.8262-25.56], p = .0488) were found to be significantly associated with disease progression. Also, rs13485C/C genotype in combination with rs919267C/T (OR = 9.406 [1.384-111], p = .0085) and rs145303329 Δ19bp (OR = 3.887 [0.826-25.5], p = .048) were found to be significantly associated with disease progression. 19 bp indel rs145303329 and its allele c.1-443_1-442insCGCCCCCAGAC[C/T]CAGGCCC from BST2 promoter also showed association with disease progression (OR = 12.97 [0.9731-850.5], p = .026). Docking of AP2 repressor with above allele showed the total binding energy of LTNPs and progressors to be -2581.42 kcal/mol and - 3563.27/ -3562.84 kcal/mol respectively. We have identified the novel association of three BST2 gene SNPs; rs919267, rs13485 and indel rs145303329 from Indian cohort to be associated with the risk of HIV-1 disease progression for the first time.
Quantitative proteomic analysis of duck ovarian follicles infected with duck tembusu virus by Label-Free LC-MS
DOI:10.3389/fmicb.2016.00463
URL
PMID:27066001
[本文引用: 1]

Duck Tembusu virus (DTMUV) is a newly emerging pathogenic flavivirus that has caused massive economic losses to the duck industry in China. DTMUV infection mainly results in significant decreases in egg production in egg-laying ducks within 1-2 weeks post infection. However, information on the comparative protein expression of host tissues in response to DTMUV infection is limited. In the present study, the cellular protein response to DTMUV infection in duck ovarian follicles was analyzed using nano-flow high-performance liquid chromatography-electrospray tandem mass spectrometry. Quantitative proteomic analysis revealed 131 differentially expressed proteins, among which 53 were up regulated and 78 were down regulated. The identified proteins were involved in the regulation of essential processes such as cellular structure and integrity, RNA processing, protein biosynthesis and modification, vesicle transport, signal transduction, and mitochondrial pathway. Some selected proteins that were found to be regulated in DTMUV-infected tissues were screened by quantitative real-time PCR to examine their regulation at the transcriptional level, western blot analysis was used to validate the changes of some selected proteins on translational level. To our knowledge, this study is the first to analyze the proteomic changes in duck ovarian follicles following DTMUV infection. The protein-related information obtained in this study may be useful to understand the host response to DTMUV infection and the inherent mechanism of DTMUV replication and pathogenicity.
樱桃谷鸭OASL基因全长cDNA的克隆及其生物信息学分析
Cloning and bioinformatics analysis of cherry valley duck oligoadenylate synthase-like gene (OASL)
TAG-EL-DIN-HASSANA H, MASAMI M, TAKASHI A. Functional analysis of duck, goose, and ostrich 2′-5′-oligoadenylate synthetase
DOI:10.1016/j.meegid.2018.04.036
URL
PMID:29715528
[本文引用: 1]

Up-to-date the flavivirus infection in avian taxa is not clearly defined. Several reports have demonstrated that many viruses belonging to Flaviviridae may cause diseases in poultry species; however, the susceptibility of other avian species is variable and still unclear. In human and mice, the 2'-5'-oligoadenylate synthetase (OAS) proteins are associated with resistance to the flavivirus infection as well as other virus infections. However, the avian OAS proteins are rarely studied. In our previous studies, we confirmed that the chicken OAS-like protein (chOASL) expressed OAS-enzymatic activity (the classical OAS/RNase L-dependent pathway) as well as the anti-flavivirus activity (the putative OAS/RNase L-independent pathway). Therefore, the current study aimed at functional analysis of avian OAS proteins from duck, goose, and ostrich. The duOASL, goOASL, and osOAS1 proteins expressed enzymatic activity as well as chOASL, whereas osOASL expressed little enzymatic activity. On the other hand, duOASL, goOASL, and osOASL possessed significant antiviral activity against West Nile virus (WNV)-replicon replication as well as chOASL, whereas osOAS1 did not. In addition, similar to chOASL, their antiviral activity was independent of RNase L activation. These results suggest that OASL is the only OAS protein in the duck and goose as well as chicken and possesses both OAS-enzymatic and anti-flavivirus activities, whereas the ostrich possesses both OAS1 and OASL proteins with sharing the functional activities, OAS-enzymatic and anti-flavivirus activities, respectively. It is of interest that the ostrich undergoes differential process in OAS gene evolution from other poultries and thus possesses different molecular mechanism in antiviral activity.
Interferon-inducible antiviral effectors
DOI:10.1038/nri2314
URL
PMID:18575461
[本文引用: 1]

Since the discovery of interferons (IFNs), considerable progress has been made in describing the nature of the cytokines themselves, the signalling components that direct the cell response and their antiviral activities. Gene targeting studies have distinguished four main effector pathways of the IFN-mediated antiviral response: the Mx GTPase pathway, the 2',5'-oligoadenylate-synthetase-directed ribonuclease L pathway, the protein kinase R pathway and the ISG15 ubiquitin-like pathway. As discussed in this Review, these effector pathways individually block viral transcription, degrade viral RNA, inhibit translation and modify protein function to control all steps of viral replication. Ongoing research continues to expose additional activities for these effector proteins and has revealed unanticipated functions of the antiviral response.
2′,5′-Oligoadenylate synthetase 1(OAS1) inhibits PRRSV replication in Marc-145 cells
DOI:10.1016/j.antiviral.2016.07.001
URL
PMID:27395032
[本文引用: 1]

Porcine reproductive and respiratory syndrome virus (PRRSV) is an economically destructive disease for global pig industry. Although its invasion mechanism is clear, the knowledge of pathogen and host interaction is less known. Here, we found that PPRSV infection led to induction of 2', 5'-oligoadenylate synthetase gene 1(OAS1), an important interferon-stimulated gene. More importantly, ectopic overexpression of OAS1 significantly restricted the replication of PRRSV in 24 and 36 h post-infection in Marc-145 cells, and indirect immunofluorescence assay with antibody against PRRSV N protein displayed significantly lower frequency of fluorescence stained cells and reduced cytopathogenic effects of PRRSV on OAS1-transfected Marc-145 cells. Meanwhile, knockdown of endogenous OAS1 increased the PRRSV ORF7 mRNA level to 1.6 and 1.7 times of that in control in 24 and 36 h post-infection of PRRSV, and led to approximate 5.5 times increase of the frequency of fluorescence positive cells compared to negative control (p < 0.01). The obtained results strongly support a direct restriction function of OAS1 to PRRSV replication, which may contribute to the antiviral effect of the interferon system on PRRSV replication.
Activation of 2’ 5’-oligoadenylate synthetase by stem loops at the 5’-end of the West Nile virus genome
DOI:10.1371/journal.pone.0092545
URL
PMID:24651762
[本文引用: 1]

West Nile virus (WNV) has a positive sense RNA genome with conserved structural elements in the 5' and 3' -untranslated regions required for polyprotein production. Antiviral immunity to WNV is partially mediated through the production of a cluster of proteins known as the interferon stimulated genes (ISGs). The 2' 5'-oligoadenylate synthetases (OAS) are key ISGs that help to amplify the innate immune response. Upon interaction with viral double stranded RNA, OAS enzymes become activated and enable the host cell to restrict viral propagation. Studies have linked mutations in the OAS1 gene to increased susceptibility to WNV infection, highlighting the importance of OAS1 enzyme. Here we report that the region at the 5'-end of the WNV genome comprising both the 5'-UTR and initial coding region is capable of OAS1 activation in vitro. This region contains three RNA stem loops (SLI, SLII, and SLIII), whose relative contribution to OAS1 binding affinity and activation were investigated using electrophoretic mobility shift assays and enzyme kinetics experiments. Stem loop I, comprising nucleotides 1-73, is dispensable for maximum OAS1 activation, as a construct containing only SLII and SLIII was capable of enzymatic activation. Mutations to the RNA binding site of OAS1 confirmed the specificity of the interaction. The purity, monodispersity and homogeneity of the 5'-end (SLI/II/III) and OAS1 were evaluated using dynamic light scattering and analytical ultra-centrifugation. Solution conformations of both the 5'-end RNA of WNV and OAS1 were then elucidated using small-angle x-ray scattering. In the context of purified components in vitro, these data demonstrate the recognition of conserved secondary structural elements of the WNV genome by a member of the interferon-mediated innate immune response.
Differential regulation of the OASL and OASl genes in response to viral infections
DOI:10.1089/jir.2008.0050
URL
PMID:19203244
[本文引用: 1]

The 2'-5' oligoadenylate synthetase (OAS) family consist of three genes encoding active OAS enzymes (OAS1-3) and an OAS-Like (OASL) gene encoding an inactive protein. The transcription of all four members of this family is actively induced by interferon (IFN), but so far no attempt to systematically analyze the expression of these genes during viral infection has been made. We analyzed the expression of the human OAS1 and OASL genes in response to infection with Sendai virus or Influenza A virus. Surprisingly, we found a marked difference in the expression pattern of these genes. Our data showed that the OASL gene is rapidly induced in response to viral infection and that this induction is mediated by IFN regulatory factor 3 (IRF-3). In contrast to the OASL gene, the induction of the OAS1 gene by virus infection was lower, and did require a functional type I IFN response. The pronounced difference in gene regulation between the OAS1 and OASL genes agrees with a functional difference between these genes, which must exist as a consequence of the lack of the 2-5A synthetase activity of the OASL protein. Furthermore, the behavior of the OASL gene is consistent with the behavior of an antiviral gene.
Molecular cloning, characterization, and expression of duck 2′-5′-oligoadenylate synthetase-like gene
DOI:10.1016/j.gene.2017.07.067
URL
PMID:28754636
[本文引用: 3]

2'-5'-Oligoadenylate synthetase-like protein (OASL) is an interferon-inducible antiviral protein that exerts antiviral effects through the RNase L- or retinoic acid-inducible gene I (RIG-I)-dependent signalling pathway. In this study, we identified and cloned the OASL gene (named duOASL) from healthy adult Cherry Valley ducks. Full-length duOASL cDNA (1630bp) encoded a 504-amino acid polypeptide containing three conserved domains, namely, nucleotidyltransferase domain, 2'-5'-oligoadenylate synthetase domain, and two ubiquitin-like repeats. DuOASL mRNA expression was quantified by performing quantitative reverse transcription-PCR (qRT-PCR). Results of qRT-PCR showed that duOASL was broadly expressed in all examined tissues, with the highest mRNA expression in the large intestine. Antiviral activity of duOASL was measured by determining its effect on Duck Tembusu virus (DTMUV) replication in vitro. We found that duOASL overexpression slightly inhibited DTMUV replication, whereas duOASL knockdown by using a specific small interfering RNA increased DTMUV replication in DF-1 cells. Thus, we successfully cloned and characterized the antiviral protein duOASL from Cherry Valley ducks and found that it exerted antiviral effects against DTMUV. These results provide a solid foundation for performing further studies to determine the mechanism underlying the antiviral effect of duOASL at the cellular level.
OASL-a new player in controlling antiviral innate immunity
DOI:10.1016/j.coviro.2015.01.010
URL
PMID:25676874
[本文引用: 1]

The cellular innate immune system plays a crucial role in mounting the initial resistance to virus infection. It is comprised of various pattern-recognition receptors that induce type I interferon production, which further shapes the adaptive immunity. However, to overcome this resistance and promote replication, viruses have evolved mechanisms to evade this host innate immune response. Here we discuss a recently described mechanism of boosting the innate immunity by oligoadenylate synthetase-like (OASL) protein, which can potentially be used to overcome viral evasion and enhance innate immunity.
Structural and functional analysis reveals that human OASL binds dsRNA to enhance RIG-I signaling
DOI:10.1093/nar/gkv389
URL
PMID:25925578
[本文引用: 1]

The oligoadenylate synthetase (OAS) enzymes are cytoplasmic dsRNA sensors belonging to the antiviral innate immune system. Upon binding to viral dsRNA, the OAS enzymes synthesize 2'-5' linked oligoadenylates (2-5As) that initiate an RNA decay pathway to impair viral replication. The human OAS-like (OASL) protein, however, does not harbor the catalytic activity required for synthesizing 2-5As and differs from the other human OAS family members by having two C-terminal ubiquitin-like domains. In spite of its lack of enzymatic activity, human OASL possesses antiviral activity. It was recently demonstrated that the ubiquitin-like domains of OASL could substitute for K63-linked poly-ubiquitin and interact with the CARDs of RIG-I and thereby enhance RIG-I signaling. However, the role of the OAS-like domain of OASL remains unclear. Here we present the crystal structure of the OAS-like domain, which shows a striking similarity with activated OAS1. Furthermore, the structure of the OAS-like domain shows that OASL has a dsRNA binding groove. We demonstrate that the OAS-like domain can bind dsRNA and that mutating key residues in the dsRNA binding site is detrimental to the RIG-I signaling enhancement. Hence, binding to dsRNA is an important feature of OASL that is required for enhancing RIG-I signaling.
What is the oligoadenylate synthetases-like protein and does it have therapeutic potential for influenza?
DOI:10.1586/17476348.2015.994608
URL
PMID:25544107
[本文引用: 1]

Besides its pandemic potential, seasonal influenza infection is associated with an estimated 250,000 to 500,000 deaths worldwide every year. Part of this virulence of influenza virus can be attributed to its ability to evade the host innate immune response. Here, we discuss the possibility of using a recently described mechanism of boosting the innate immunity by oligoadenylate synthetase-like protein, to combat influenza infections.
2'-5'-Oligoadenylate synthetase-like protein inhibits respiratory syncytial virus
DOI:10.1128/JVI.01076-15
URL
PMID:26178980
[本文引用: 1]

2'-5'-Oligoadenylate synthetase-like protein (OASL) is an interferon-inducible antiviral protein. Here we describe differential inhibitory activities of human OASL and the two mouse OASL homologs against respiratory syncytial virus (RSV) replication. Interestingly, nonstructural protein 1 (NS1) of RSV promoted proteasome-dependent degradation of specific OASL isoforms. We conclude that OASL acts as a cellular antiviral protein and that RSV NS1 suppresses this function to evade cellular innate immunity and allow virus growth.
E. Origin and development of oligoadenylate synthetase immune system
DOI:10.1186/s12862-018-1315-x
URL
PMID:30587119
[本文引用: 1]

Oligoadenylate synthetases (OASs) are widely distributed in Metazoa including sponges, fish, reptiles, birds and mammals and show large variation, with one to twelve members in any given species. Upon double-stranded RNA (dsRNA) binding, avian and mammalian OASs generate the second messenger 2'-5'-linked oligoadenylate (2-5A), which activates ribonuclease L (RNaseL) and blocks viral replication. However, how Metazoa shape their OAS repertoires to keep evolutionary balance to virus infection is largely unknown. We performed comprehensive phylogenetic and functional analyses of OAS genes from evolutionarily lower to higher Metazoa to demonstrate how the OAS repertoires have developed anti-viral activity and diversified their functions.
Molecular cloning of porcine 2′,5′-oligoadenylate synthetase-like protein and its role in porcine reproductive and respiratory syndrome virus infection
DOI:10.1016/j.micpath.2018.09.023
URL
PMID:30240816
[本文引用: 1]

Porcine 2',5'-oligoadenylate synthetase-like protein is an essential antiviral protein induced by interferons; however, its bioinformatics, genetic characteristics and immunological characteristics related to porcine reproductive and respiratory syndrome virus are still unknown. In this study, porcine 2',5'-oligoadenylate synthetase-like protein was cloned, and various attributes were predicted by bioinformatics analysis. Through RNAi depletion and overexpression methods, it was determined that porcine OASL not only inhibits porcine reproductive and respiratory virus replication but also activates interferon-beta production and the interferon-beta promoter, promoting the expression of interferon-beta mRNA. Through the depletion of different amino acids at the N and C termini, the antiviral activity and promoting the activity of interferon beta were evaluated. The results demonstrated that 31-60 amino acids at the N terminus were critical for virus replication. This study laid a theoretical foundation for exploring the characteristics of the porcine 2',5'-oligoadenylate synthetase-like protein and suggested a new strategy for the prevention and control of porcine reproductive and respiratory syndrome virus and investigation of the therapeutic mechanism of this protein.
Molecular characterization of the 2′,5 -oligoadenylate synthetase family in the Chinese tree shrew ( Tupaia Belangeri Chinensis)
DOI:10.1016/j.cyto.2018.11.009
URL
PMID:30467096
[本文引用: 1]

Virus infection induces type I interferons (IFNs) that in turn exert their pleiotropic effects through inducing a large number of interferon-stimulated genes (ISGs). The IFN-induced 2',5'-oligoadenylate synthetases (OASs) have been identified as a member of the ISGs family characterized by the ability to synthesize 2',5'-oligoadenylate (2-5A), which can induce the degradation of viral RNA by activating RNase L within the infected cells to block viral replications. In this study, we characterized the OASs of the Chinese tree shrew (Tupaia belangeri chinensis), a small mammal genetically close to primates and has the potential as animal model for viral infections. We identified 4 putative tree shrew OASs (tOASs, including tOAS1, tOAS2, tOASL1, and tOASL2) and characterized their roles in antiviral responses. Tree shrew lost tOAS3 that was presented in human and mouse. Phylogenetic analyses based on the protein sequences showed a close relationship of tOASs with those of mammals. Constitutive mRNA expression of tOASs was found in seven tissues (heart, liver, spleen, lung, kidney, small intestine and brain). Moreover, tOASs were significantly up-regulated upon various virus infections. Overexpression of tOASs significantly inhibited DNA virus and RNA virus replications in tree shrew primary renal cells. tOAS1 and tOAS2, but not tOASL1 and tOASL2, exerted their anti-HSV activity in an RNase L-dependent pathway. Collectively, our results revealed the evolutionary conservation of tOASs in tree shrew and might offer helpful information for creating viral infection models using the Chinese tree shrew.
The 2′-5′-oligoadenylate synthetase in the lowest metazoan: isolation, cloning, expression and functional activity in the sponge
Lubomirskia baicalensis
DOI:10.1016/j.molimm.2007.07.036
URL
[本文引用: 1]

Abstract
Aquatic animals, especially filter feeders such as sponges [phylum Porifera], are exposed to a higher viral load than terrestrial species. Until now, the antiviral defense system in the evolutionary oldest multicellular organisms, sponges, is not understood. One powerful protection of vertebrates against virus infection is mediated by the interferon (IFN)-inducible 2′-5′-oligoadenylate synthetase [(2-5)A synthetase] system. In the present study we cloned from the freshwater sponge Lubomirskia baicalensis a cDNA encoding a 314 aa long ORF with a calculated size of 35748 Da, a putative (2-5)A synthetase, and raised antibodies against the recombinant protein. The native enzyme was identified in a crude extract from L. baicalensis by application of a novel separation procedure based on polymer coated ferromagnetic nanoparticles. The particles were derivatized with a synthetic double-stranded RNA [dsRNA], synthetic poly(I:C), a known allosteric activator of the latent (2-5)A synthetase. These particles were used to separate a single 35 kDa protein from a crude extract of L. baicalensis, which cross-reacted with antibodies raised against the sponge enzyme. In situ hybridization studies revealed that highest expression of the gene is seen in cells surrounding the aquiferous canals. Finally primmorphs, an in vitro cell culture system, from L. baicalensis were exposed to poly(I:C); they responded to this dsRNA with an increased expression of the (2-5)A synthetase gene already after a 1-day incubation period. We conclude that sponges contain the (2-5)A synthetase antiviral protection system.
The oligoadenylate synthetase family: An ancient protein family with multiple antiviral activities
DOI:10.1089/jir.2010.0107
URL
[本文引用: 1]

The 2'-5' oligoadenylate synthetases (OAS) are interferon-induced antiviral enzymes that recognize virally produced dsRNA and initiate RNA destabilization through activation of RNase L within infected cells. However, recent evidence points toward several RNase L-independent pathways, through which members of the OAS family can exert antiviral activity. The crystal structure of OAS led to a novel insight into the catalytic mechanism, and revealed a remarkable similarity between OAS, Polyadenosine polymerase, and the class I CCA-adding enzyme from Archeoglobus fulgidus. This, combined with a variety of bioinformatic data, leads to the definition of a superfamily of template independent polymerases and proved that the OAS family are ancient proteins, which probably arose as early as the beginning of metazoan evolution.
OAS蛋白结构与抗病毒机制关系的研究进展
Advances in the study of the relationship between the structure of OAS protein and the antiviral mechanism
Interferon-inducible oligoadenylate synthetase-like protein acts as an antiviral effector against classical swine fever virus via the MDA5-mediated type I interferon signaling pathway
DOI:10.1128/JVI.01514-16
URL
PMID:28331099
[本文引用: 1]

Classical swine fever virus (CSFV) is the causative agent of classical swine fever (CSF), which poses a serious threat to the global pig industry. Interferons (IFNs) and IFN-stimulated genes (ISGs) play a key role in host antiviral defense. We have previously screened the porcine 2'-5'-oligoadenylate synthetase-like protein (pOASL) as a potential anti-CSFV ISG using a reporter CSFV. This study aimed to clarify the underlying antiviral mechanism of pOASL against CSFV. We confirmed that CSFV replication was significantly suppressed in lentivirus-delivered, pOASL-overexpressing PK-15 cells, whereas silencing the expression of endogenous pOASL by small interfering RNAs markedly enhanced CSFV growth. In addition, the transcriptional level of pOASL was upregulated both in vitro and in vivo upon CSFV infection. Interestingly, the anti-CSFV effects of pOASL are independent of the canonical RNase L pathway but depend on the activation of the type I IFN response. Glutathione S-transferase pulldown and coimmunoprecipitation assays revealed that pOASL interacts with MDA5, a double-stranded RNA sensor, and further enhances MDA5-mediated type I IFN signaling. Moreover, we showed that pOASL exerts anti-CSFV effects in an MDA5-dependent manner. In conclusion, pOASL suppresses CSFV replication via the MDA5-mediated type I IFN-signaling pathway.IMPORTANCE The host innate immune response plays an important role in mounting the initial resistance to viral infection. Here, we identify the porcine 2'-5'-oligoadenylate synthetase-like protein (pOASL) as an interferon (IFN)-stimulated gene (ISG) against classical swine fever virus (CSFV). We demonstrate that the anti-CSFV effects of pOASL depend on the activation of type I IFN response. In addition, we show that pOASL, as an MDA5-interacting protein, is a coactivator of MDA5-mediated IFN induction to exert anti-CSFV actions. This work will be beneficial to the development of novel anti-CSFV strategies by targeting pOASL.
猪源OAS抗日本脑炎病毒感染的活性研究
[D].
Investigation on antiviral effect of porcine 2′-5′ oligoadenylate synthetases on JEV replication
[D].
Oligoadenylate synthase-like (OASL) proteins: dual functions and associations with diseases
DOI:10.1097/BOT.0000000000001718
URL
PMID:31842188
[本文引用: 1]

To develop a tool that can be used pre-operatively to identify patients at risk of poor functional outcome following operative repair of fracture nonunion.
OASL1 inhibits translation of the type I interferon-regulating transcription factor IRF7
DOI:10.1038/ni.2535
URL
[本文引用: 1]

The production of type I interferon is essential for viral clearance but is kept under tight control to avoid unnecessary tissue damage from hyperinflammatory responses. Here we found that OASL1 inhibited translation of IRF7, the master transcription factor for type I interferon, and thus negatively regulated the robust production of type I interferon during viral infection. OASL1 inhibited the translation of IRF7 mRNA by binding to the 5' untranslated region (UTR) of IRF7 and possibly by inhibiting scanning of the 43S preinitiation complex along the message. Oasl1(-/-) mice were resistant to viral infection because of the greater abundance of type I interferon, which suggests that OASL1 could be a potential therapeutic target for boosting the production of type I interferon during viral infection.
Evolution of the 2′-5′-oligoadenylate synthetase family in eukaryotes and bacteria
DOI:10.1007/s00239-009-9299-1
URL
[本文引用: 1]

The 2′-5′-oligoadenylate synthetase (OAS) belongs to a nucleotidyl transferase family that includes poly(A) polymerases and CCA-adding enzymes. In mammals and birds, the OAS functions in the interferon system but it is also present in an active form in sponges, which are devoid of the interferon system. In view of these observations, we have pursued the idea that OAS genes could be present in other metazoans and in unicellular organisms as well. We have identified a number of OAS1 genes in annelids, mollusks, a cnidarian, chordates, and unicellular eukaryotes and also found a family of proteins in bacteria that contains the five OAS-specific motifs. This indicates a specific relationship to OAS. The wide distribution of the OAS genes has made it possible to suggest how the OAS1 gene could have evolved from a common ancestor to choanoflagellates and metazoans. Furthermore, we suggest that the OASL may have evolved from an ancestor of cartilaginous fishes, and that the OAS2 and the OAS3 genes evolved from a mammalian ancestor. OAS proteins function in the interferon system in mammals. This system is only found in jawed vertebrates. We therefore suggest that the original function of OAS may differ from its function in the interferon system, and that this original function of OAS is preserved even in OAS genes that code for proteins, which do not have 2′-5′-oligoadenylate synthetase activity.
Three-dimensional models of human 2′-5′-oligoadenylate synthetases: a new computational method for reconstructing an enzyme assembly