













中国农业科学 ›› 2021, Vol. 54 ›› Issue (14): 3029-3042.doi: 10.3864/j.issn.0578-1752.2021.14.009
收稿日期:2020-11-06
接受日期:2021-01-15
出版日期:2021-07-16
发布日期:2021-07-26
通讯作者:
尤民生,何玮毅
作者简介:李飞飞,E-mail: 基金资助:
LI FeiFei( ),WANG BeiBei,LAI YingFang,YANG FeiYing,YOU MinSheng(
),WANG BeiBei,LAI YingFang,YANG FeiYing,YOU MinSheng( ),HE WeiYi(
),HE WeiYi( )
)
Received:2020-11-06
Accepted:2021-01-15
Online:2021-07-16
Published:2021-07-26
Contact:
MinSheng YOU,WeiYi HE
摘要:
【目的】RNA甲基化是基因转录后水平表观修饰的主要形式,参与了众多重要的细胞学过程。小菜蛾(Plutella xylostella)是危害十字花科蔬菜的重要寡食性害虫,与RNA甲基化相关基因的功能尚未见报道。本研究通过克隆小菜蛾的RNA甲基化蛋白同源基因fl(2)d,鉴定其表达模式,并敲除该基因以探究其生物学功能。【方法】通过小菜蛾基因组网站查找fl(2)d基因序列,PCR扩增其蛋白质编码序列(CDS);采用实时荧光定量PCR(qRT-PCR)技术,检测小菜蛾不同发育阶段个体以及成虫生殖腺中fl(2)d的相对表达量;运用CRISPR/Cas9结合卵的显微注射技术,对小菜蛾fl(2)d进行编辑;将fl(2)d被编辑过的成虫与野生型成虫杂交,并对其产生的后代进行近交,筛选fl(2)d突变品系;观测并比较突变体与野生型个体遗传特性、生物学参数和表型的差异,明确fl(2)d的功能。【结果】克隆得到长度为912 bp的fl(2)d CDS,fl(2)d在雌蛹、雌成虫和卵中的表达量较高,雄成虫和雄蛹的表达量较低,幼虫期的表达量最低,成虫卵巢中表达量显著高于精巢。通过向小菜蛾的卵注射靶向fl(2)d的向导RNA(sgRNA)和Cas9蛋白的混合物,对所产生的阳性后代进行10代的单对近交筛选,获得3种杂合的移码突变品系,分别缺失了4个(Δfl(2)d213-4)、5个(Δfl(2)d213-5)和7个(Δfl(2)d214-7)碱基。在上述品系的筛选过程中,发现了6只缺失4个碱基的纯合突变个体,2只缺失5个碱基的纯合突变个体;缺失4个碱基的纯合个体成功配成了两对,近交未产卵;剩余的2只缺失4个碱基的雄性纯合个体和2只缺失5个碱基的雄性纯合个体分别与同世代的雌性杂合突变个体近交后仍未产卵。说明fl(2)d纯合突变的个体存活率极低,且可能无法产生后代。通过分析后代基因型的分离比,发现杂合突变个体近交以及杂合突变个体与野生型个体杂交产生的后代中,杂合突变个体与野生型个体的比例分别略小于2和1,说明fl(2)d杂合突变会影响小菜蛾正常的生长发育,并导致部分个体死亡。杂合突变体后代中含有突变的雌雄个体比例接近1﹕1(P<0.05),推测小菜蛾fl(2)d可能与性别决定无关。只要是有突变品系小菜蛾所参与的交配,雌成虫产卵量和卵的孵化率均显著低于野生型(P<0.01),所产的卵多数发育异常,表现为失水皱缩、不能正常孵化。通过对成虫的生殖腺进行解剖,发现在野生型雌成虫与突变体雄成虫交配后,卵巢内卵的附着量较未交配的个体明显减少;未交配的突变体雌成虫卵巢内卵的附着量亦少于野生型,而突变体雄成虫的精巢未见明显异常。部分能够孵化的杂合突变个体在整个发育过程会发生不同程度的畸变,导致不能正常完成整个世代;另外一些杂合突变个体未见异常,可以将突变类型遗传给后代。根据上述发现,提出了基于fl(2)d的小菜蛾遗传防控模型。【结论】fl(2)d参与小菜蛾的生殖过程和胚胎发育,突变后显著影响后代种群数量,是开展小菜蛾遗传控制的理想靶标。
李飞飞,王贝贝,赖颖芳,杨菲颖,尤民生,何玮毅. fl(2)d单等位基因的敲除显著降低小菜蛾的生殖力和育性[J]. 中国农业科学, 2021, 54(14): 3029-3042.
LI FeiFei,WANG BeiBei,LAI YingFang,YANG FeiYing,YOU MinSheng,HE WeiYi. Knockout of Single Allele of fl(2)d Significantly Decreases the Fecundity and Fertility inPlutella xylostella[J]. Scientia Agricultura Sinica, 2021, 54(14): 3029-3042.
表1
本研究所用引物序列"
| 引物名称Primer name | 序列Sequence (5′-3′) | 用途Usage |
|---|---|---|
| Fl(2)d-ORF-F | CGAGGAGACAGAACGCCTT | 克隆fl(2)d Cloning offl(2)d |
| Fl(2)d-ORF-R | GGTGACGGTGAGGGGTTC | |
| qPCR-Fl(2)d-F | CGGAGCTCAAATCATCACACGC | fl(2)d定量表达 qRT-PCR of fl(2)d |
| qPCR-Fl(2)d-R | GCCCAGGTCCTCATTCTCCT | |
| RPL32-F | CAATCAGGCCAATTTACCGC | 内参基因RPL32定量表达 qRT-PCR of reference RPL32 |
| RPL32-R | CTGGGTTTACGCCAGTTACG | |
| Fl(2)d-Mut-TF | GTATAATCCAATGAAGGTATGACAG | 突变检测 Mutation detection |
| Fl(2)d-Mut-TR | CCTACAGTGAAACCCGCAA | |
| CRISPR-F1 | TAATACGACTCACTATAGGAAGCCTGGAAAAGGCGAAAGGTTTTAGAGCTAGAAATAGCAAGTTAAAATAAGGCTAGTCC | 合成sgRNA Synthesis of sgRNAs |
| CRISPR-F2 | TAATACGACTCACTATAGGAAGGCTAGCAGCTAAAGAACGTTTTAGAGCTAGAAATAGCAAGTTAAAATAAGGCTAGTCC | |
| CRISPR-R | AAAAGCACCGACTCGGTGCCACTTTTTCAAGTTGATAACGGACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAA |
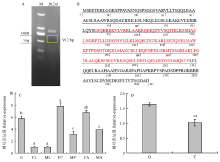
图2
小菜蛾fl(2)d克隆及表达模式 A:fl(2)d CDS序列的克隆CDS cloning of fl(2)d;M:DNA分子标准DNA marker。B:预测的fl(2)d蛋白质序列Predicted protein sequence of fl(2)d;红色字母:保守功能域Red letters indicate the conserved domain。C:fl(2)d在不同发育阶段的表达Expression of fl(2)d in different developmental stages;E:卵Egg;FL:雌幼虫Female larva;ML:雄幼虫Male larva;FP:雌蛹Female pupa;MP:雄蛹Male pupa;FA:雌成虫Female adult;MA:雄成虫Male adult;采用单因素方差分析法进行差异显著性检验,使用Tukey法进行多重比较,不同字母表示差异显著(P<0.05)Significant difference analysis was performed using one-way ANOVA followed by a Tukey’s HSD post hoc test. Different letters indicate significant difference atP<0.05 level。D:fl(2)d在成虫生殖腺的表达Expression offl(2)d in adult gonads;O:卵巢Ovary;T:精巢Testis;采用t检验进行差异显著性分析,**表示检验性水平P<0.01 Significant difference analysis was performed usingt test. Double asterisks indicate significant difference of P<0.01 "
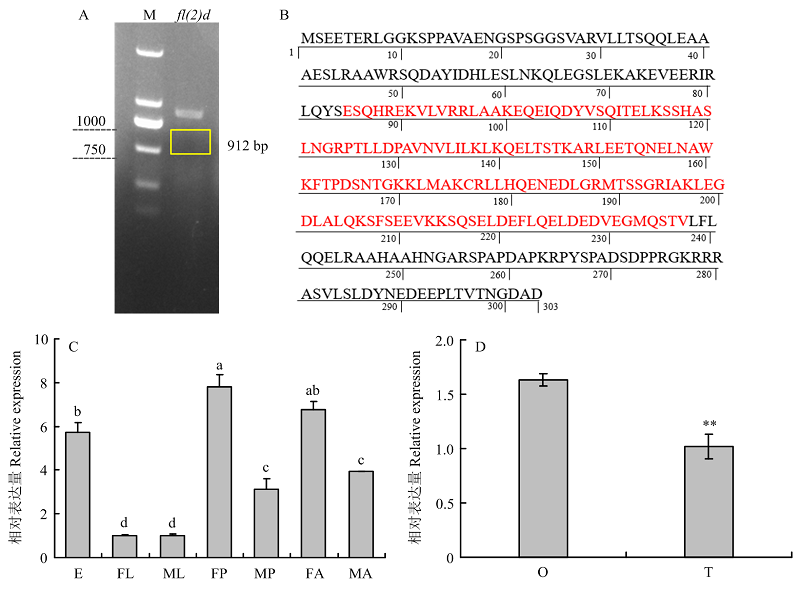
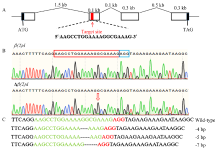
图3
小菜蛾fl(2)d基因的编辑 A:fl(2)d基因结构及sgRNA靶点示意图Schematic diagram of the fl(2)d gene structure and sgRNA target site。B:G0代野生型(上)和突变体(下)成虫fl(2)d测序峰图;红色框是sgRNA,蓝色框是PAM结构Sequencing chromatograms of fl(2)d of wild-type (top) and mutant (bottom) adults; The edited site is indicated by a red rectangle and the protospacer adjacent motif (PAM) sequence is showed by a blue rectangle。C:突变序列,虚线表示缺失的序列Mutant sequences, the dashed lines represent the deleted bases caused by CRISPR/Cas9 "
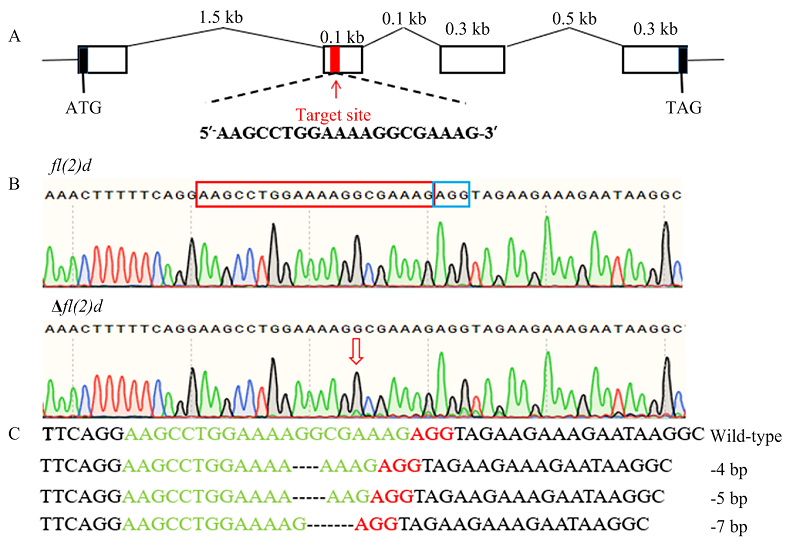
表2
G2—G10代fl(2)d基因型分离情况 "
| G2代至G10代不同基因型个体数 Numbers of different genotypes from G2 to G10 | AA×Aa(亲本基因型Parental genotype) | Aa×Aa(亲本基因型Parental genotype) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Δfl(2)d-4 | Δfl(2)d-5 | Δfl(2)d-7 | P=0.05, df=1, χ2=3.841 | Δfl(2)d-4 | Δfl(2)d-5 | Δfl(2)d-7 | P=0.05, df=1, χ2=3.841 | ||||||||||||
| AA | Aa | AA | Aa | AA | Aa | Aa/AA | χ2 | AA | Aa | aa | AA | Aa | aa | AA | Aa | aa | Aa/AA | χ2 | |
| G2 | 13 | 13 | 11 | 11 | 22 | 20 | 0.957 | 0.044 | 6 | 16 | 0 | / | / | / | 3 | 9 | 0 | 2.778 | 0.721 |
| G3 | 19 | 19 | / | / | 40 | 30 | 0.831 | 0.926 | 15 | 24 | 3 | 7 | 5 | 2 | 10 | 16 | 0 | 1.406 | 2.506 |
| G4 | / | / | / | / | 14 | 14 | 1.000 | 0 | 7 | 16 | 3 | 14 | 30 | 0 | 11 | 19 | 0 | 2.031 | 0.095 |
| G5 | 20 | 14 | 25 | 37 | 1 | 3 | 1.174 | 0.640 | / | / | / | 11 | 17 | 0 | 13 | 23 | 0 | 1.667 | 0.500 |
| G6 | 5 | 3 | 23 | 21 | 20 | 8 | 0.667 | 3.200 | / | / | / | 15 | 25 | 0 | 2 | 6 | 0 | 1.824 | 0.094 |
| G7 | 17 | 7 | 18 | 12 | 19 | 15 | 0.630 | 4.545 | / | / | / | 2 | 10 | 0 | / | / | / | 5.000 | 2.063 |
| G8 | 6 | 6 | 6 | 4 | 19 | 15 | 0.806 | 0.643 | / | / | / | 3 | 7 | 0 | 1 | 3 | 0 | 2.500 | 0.339 |
| G9 | / | / | 7 | 9 | 11 | 9 | 1.000 | 0 | / | / | / | / | / | / | / | / | / | / | / |
| G10 | / | / | / | / | 18 | 26 | 1.444 | 1.455 | / | / | / | 3 | 5 | 0 | / | / | / | 1.667 | 0.031 |
| 合计Total | 80 | 62 | 90 | 94 | 164 | 140 | 0.886 | 0.292 | 28 | 56 | 6 | 55 | 99 | 2 | 40 | 76 | 0 | 1.878 | 0.488 |
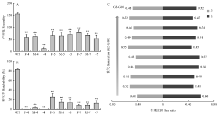
图4
fl(2)d基因突变对小菜蛾产卵量、孵化率和性别比例的影响 A:突变品系的产卵量The fecundity of mutant strains。B:突变品系的孵化率The hatchability of mutant strains。WT:野生型成虫杂交Hybridization between wild-type adults;F-4:杂合雌性Δfl(2)d213-4成虫与野生型杂交Hybridization of heterozygous female adult of Δ fl(2)d213-4 with wild-type;M-4:杂合雄性Δfl(2)d213-4成虫与野生型杂交Hybridization of heterozygous male adult of Δ fl(2)d213-4 with wild-type;-4:杂合Δfl(2)d213-4成虫近交Inbreeding between heterozygous adults of Δ fl(2)d213-4;F-5:杂合雌性Δfl(2)d213-5成虫与野生型杂交Hybridization of heterozygous female adult of Δ fl(2)d213-5 with wild-type;M-5:杂合雄性Δfl(2)d213-5成虫与野生型杂交Hybridization of heterozygous male adult of Δ fl(2)d213-5 with wild-type;-5:杂合Δfl(2)d213-5成虫近交Inbreeding between heterozygous adults of Δ fl(2)d213-5;F-7:杂合雌性Δfl(2)d214-7成虫与野生型杂交Hybridization of heterozygous female adult of Δ fl(2)d214-7 with wild-type;M-7:杂合雄性Δfl(2)d214-7成虫与野生型杂交Hybridization of heterozygous male adult of Δ fl(2)d214-7 with wild-type;-7:杂合Δfl(2)d214-7成虫近交Inbreeding between heterozygous adults of Δ fl(2)d214-7。C:性别比例The sex ratios of mutant offspring;采用t检验进行差异显著性分析,**表示显著性水平P<0.01 Significant difference analysis was performed usingt test. Double asterisks indicate significant difference of P<0.01 "
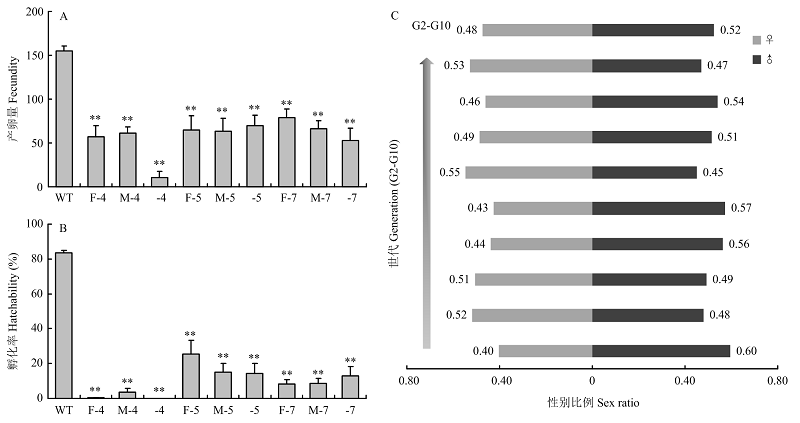

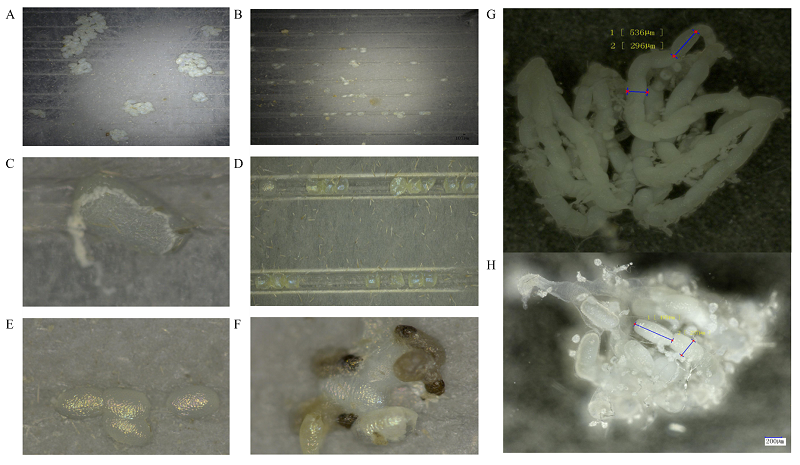
图5
小菜蛾fl(2)d突变体与野生型雌成虫生殖表型比较 A:野生型雌成虫产卵情况The fecundity of wild-type female adult。B:突变体雌成虫产卵情况The fecundity of mutant female adult。C、D:失水皱缩的卵The shrinking and dehydrated eggs。E:未能正常孵化的卵Eggs that do not hatch successfully。F:未能正常出卵的幼虫The larvae that fail to come out of eggs。G:未交配野生型雌成虫卵巢Ovary of the wild-type virgin female adult。H:与突变体雄成虫交配后野生型雌成虫卵巢Ovary of the wild-type female adult mated with the mutant male adult。除A图外,所观察的卵均为突变体雌成虫与野生型雄成虫单对交配后所产的后代,突变体类型为Δfl(2)d213-5 Except for figure A, the presented phenotypes of the eggs are all produced from the single-pair mating of mutant female adult and wild-type male adult, and the type of mutation is Δ fl(2)d213-5 "
| [1] |
FURLONG M J, WRIGHT D J, DOSDALL L M. Diamondback moth ecology and management: Problems, progress, and prospects. Annual Reviews of Entomology, 2013, 58:517-541.
doi: 10.1146/annurev-ento-120811-153605 |
| [2] |
LI Z Y, FENG X, LIU S S, YOU M S, FURLONG M J. Biology, ecology, and management of the diamondback moth in China. Annual Review of Entomology, 2016, 61:277-296.
doi: 10.1146/annurev-ento-010715-023622 |
| [3] |
GURR G M, REYNOLDS O L, JOHNSON A C, DESNEUX N, ZALUCKI M P, FURLONG M J, LI Z Y, AKUTSE K S, CHEN J H, GAO X W, YOU M S. Landscape ecology and expanding range of biocontrol agent taxa enhance prospects for diamondback moth management. A review. Agronomy for Sustainable Development, 2018, 38:23.
doi: 10.1007/s13593-018-0500-z |
| [4] |
曾宝胜, 许军, 陈树清, 谭安江, 黄勇平. 昆虫种群的遗传调控. 中国科学: 生命科学, 2013, 43(12):1098-1104.
doi: 10.1360/052013-315 |
|
ZENG B S, XU J, CHEN S Q, TAN A J, HUANG Y P. Genetic regulation of insect populations. Scientia Sinica Vitae, 2013, 43(12):1098-1104. (in Chinese)
doi: 10.1360/052013-315 |
|
| [5] |
徐雪娇, 何玮毅, 杨婕, 陈玮, 尤民生. 害虫遗传防控技术的研究与运用. 中国科学: 生命科学, 2019, 49(8):938-950.
doi: 10.1360/N052018-00256 |
|
XU X J, HE W Y, YANG J, CHEN W, YOU M S. Research and applications of genetics-based methods for pest control. Scientia Sinica Vitae, 2019, 49(8):938-950. (in Chinese)
doi: 10.1360/N052018-00256 |
|
| [6] | CHEN W, YANG F Y, XU X J, KUMAR U, HE W Y, YOU M S. Genetic control of Plutella xylostella in omics era. Archives of Insect Biochemistry and Physiology, 2019, 102(3):e21621. |
| [7] |
SHELTON A M, LONG S J, WALKER A S, BOLTON M, COLLINS H L, REVUELTA L, JOHNSON L M, MORRISON N I. First field release of a genetically engineered, self-limiting agricultural pest insect: Evaluating its potential for future crop protection. Frontiers in Bioengineering and Biotechnology, 2020, 7:482.
doi: 10.3389/fbioe.2019.00482 |
| [8] |
YOU M S, YUE Z, HE W Y, YANG X H, YANG G, XIE M, ZHAN D L, BAXTER S W, VASSEUR L, GURR G M, et al. A heterozygous moth genome provides insights into herbivory and detoxification. Nature Genetics, 2013, 45(2):220-225.
doi: 10.1038/ng.2524 |
| [9] |
JIN L, WALKER A S, FU G L, HARVEY-SAMUEL T, DAFA’ALLA T, MILES A, MARUBBI T, GRANVILLE D, HUMPHREY-JONES N, O’CONNELL S, MORRISON N I, ALPHEY L. Engineered female-specific lethality for control of pest Lepidoptera. ACS Synthetic Biology, 2013, 2(3):160-166.
doi: 10.1021/sb300123m |
| [10] |
HARVEY-SAMUEL T, MORRISON N I, WALKER A S, MARUBBI T, YAO J, COLLINS H L, GORMAN K, DAVIES T G, ALPHEY N, WARNER S, SHELTON A M, ALPHEY L. Pest control and resistance management through release of insects carrying a male-selecting transgene. BMC Biology, 2015, 13:49.
doi: 10.1186/s12915-015-0161-1 |
| [11] |
PAN T. N6-methyl-adenosine modification in messenger and long non-coding RNA. Trends in Biochemical Sciences, 2013, 38(4):204-209.
doi: 10.1016/j.tibs.2012.12.006 |
| [12] |
ROUNDTREE I A, EVANS M E, PAN T, HE C. Dynamic RNA modifications in gene expression regulation. Cell, 2017, 169(7):1187-1200.
doi: 10.1016/j.cell.2017.05.045 |
| [13] |
BATISTA P J, MOLINIE B, WANG J, QU K, ZHANG J J, LI L J, BOULEY D M, LUJAN E, HADDAD B, DANESHVAR K, et al. m6A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell, 2014, 15(6):707-719.
doi: 10.1016/j.stem.2014.09.019 |
| [14] |
FU Y, DOMINISSINI D, RECHAVI G, HE C. Gene expression regulation mediated through reversible m6A RNA methylation. Nature Reviews. Genetics, 2014, 15(5):293-306.
doi: 10.1038/nrg3724 |
| [15] |
DOMINISSINI D, MOSHITCH-MOSHKOVITZ S, SCHWARTZ S, SALMON-DIVON M, UNGAR L, OSENBERG S, CESARKAS K, JACOB-HIRSCH J, AMARIGLIO N, KUPIEC M, SOREK R, RECHAVI G. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature, 2012, 485(7397):201-206.
doi: 10.1038/nature11112 |
| [16] |
MEYER K D, SALETORE Y, ZUMBO P, ELEMENTO O, MASON C E, JAFFREY S R. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell, 2012, 149(7):1635-1646.
doi: 10.1016/j.cell.2012.05.003 |
| [17] |
DOMINISSINI D, MOSHITCH-MOSHKOVITZ S, SALMON- DIVON M, AMARIGLIO N, RECHAVI G. Transcriptome-wide mapping of N6-methyladenosine by m6A-seq based on immunocapturing and massively parallel sequencing. Nature Protocols, 2013, 8(1):176-189.
doi: 10.1038/nprot.2012.148 |
| [18] |
WU B X, LI L, HUANG Y, MA J, MIN J B, MIN J R. Readers, writers and erasers of N6-methylated adenosine modification. Current Opinion in Structural Biology, 2017, 47:67-76.
doi: 10.1016/j.sbi.2017.05.011 |
| [19] |
ZHOU Z L, LICKLIDER L J, GYGI S P, REED R. Comprehensive proteomic analysis of the human spliceosome. Nature, 2002, 419(6903):182-185.
doi: 10.1038/nature01031 |
| [20] |
HORIUCHI K, KAWAMURA T, IWANARI H, OHASHI R, NAITO M, KODAMA T, HAMAKUBO T. Identification of Wilms’ tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. Journal of Biological Chemistry, 2013, 288(46):33292-33302.
doi: 10.1074/jbc.M113.500397 |
| [21] |
LIU J Z, YUE Y N, HAN D L, WANG X, FU Y, ZHANG L, JIA G F, YU M, LU Z K, DENG X, DAI Q, CHEN W Z, HE C. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nature Chemical Biology, 2014, 10(2):93-95.
doi: 10.1038/nchembio.1432 |
| [22] |
BANSAL H, YIHUA Q, IYER S P, GANAPATHY S, PROIA D, PENALVA L O, UREN P J, SURESH U, CAREW J S, KARNAD A B, WEITMAN S, TOMLINSON G E, RAO M K, KORNBLAU S M, BANSAL S. WTAP is a novel oncogenic protein in acute myeloid leukemia. Leukemia, 2014, 28(5):1171-1174.
doi: 10.1038/leu.2014.16 |
| [23] |
JIANG T, LI J S, QIAN P, XUE P, XU J, CHEN Y R, ZHU J, TANG S M, ZHAO Q L, QIAN H Y, SHEN X J. The role of N6- methyladenosine modification on diapause in silkworm (Bombyx mori) strains that exhibit different voltinism. Molecular Reproduction and Development, 2019, 86(12):1981-1992.
doi: 10.1002/mrd.v86.12 |
| [24] |
LI B Q, WANG X Y, LI Z Q, LU C C, ZHANG Q, CHANG L, LI W S, CHENG T C, XIA Q Y, ZHAO P. Transcriptome-wide analysis of N6-methyladenosine uncovers its regulatory role in gene expression in the LepidopteranBombyx mori. Insect Molecular Biology, 2019, 28(5):703-715.
doi: 10.1111/imb.v28.5 |
| [25] |
WANG M, XIAO Y, LI Y, WANG X Y, QI S Z, WANG Y, ZHAO L W, WANG K, PENG W J, LUO G Z, XUE X F, JIA G F, WU L M. RNA m6A modification functions in larval development and caste differentiation in honeybee (Apis mellifera). Cell Reports, 2021, 34(1):108580.
doi: 10.1016/j.celrep.2020.108580 |
| [26] | GUO J, TANG H W, LI J, PERRIMON N, YAN D. Xio is a component of theDrosophila sex determination pathway and RNA N6-methyladenosine methyltransferase complex. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(14):3674-3679. |
| [27] |
HAUSSMANN I U, BODI Z, SANCHEZ-MORAN E, MONGAN N P, ARCHER N, FRAY R G, SOLLER M. m6A potentiates Sxl alternative pre-mRNA splicing for robust Drosophilasex determination. Nature, 2016, 540(7632):301-304.
doi: 10.1038/nature20577 |
| [28] |
LENCE T, AKHTAR J, BAYER M, SCHMID K, SPINDLER L, HO C H, KREIM N, ANDRADE-NAVARRO M A, POECK B, HELM M, ROIGNANT J Y. m6A modulates neuronal functions and sex determination in Drosophila. Nature, 2016, 540(7632):242-247.
doi: 10.1038/nature20568 |
| [29] |
GRANADINO B, SAN JUAN A, SANTAMARIA P, SANCHEZ L. Evidence of a dual function infl(2)d, a gene needed forsex-lethal expression in Drosophila melanogaster. Genetics, 1992, 130(3):597-612.
doi: 10.1093/genetics/130.3.597 |
| [30] |
ORTEGA A, NIKSIC M, BACHI A, WILM M, SANCHEZ L, HASTIE N, VALCARCEL J. Biochemical function of female-lethal (2)D/Wilms’ tumor suppressor-1-associated proteins in alternative pre-mRNA splicing. Journal of Biological Chemistry, 2003, 278(5):3040-3047.
doi: 10.1074/jbc.M210737200 |
| [31] |
GRANADINO B, CAMPUZANO S, SANCHEZ L. The Drosophila melanogaster fl(2)dgene is needed for the female-specific splicing ofsex-lethal RNA. The EMBO Journal, 1990, 9(8):2597-2602.
doi: 10.1002/embj.1990.9.issue-8 |
| [32] |
GRANADINO B, PENALVA L O, SANCHEZ L. The gene fl(2)d is needed for the sex-specific splicing of transformer pre-mRNA but not fordouble-sex pre-mRNA in Drosophila melanogaster. Molecular and General Genetics, 1996, 253(1/2):26-31.
doi: 10.1007/s004380050292 |
| [33] |
BURNETTE J M, HATTON A R, LOPEZ A J. Trans-acting factors required for inclusion of regulated exons in the Ultrabithorax mRNAs of Drosophila melanogaster. Genetics, 1999, 151(4):1517-1529.
doi: 10.1093/genetics/151.4.1517 |
| [34] |
ORTEGA A. Localization of the Drosophilaprotein fl(2)d in somatic cells and female gonads. Cell and Tissue Research, 2005, 320(2):361-367.
doi: 10.1007/s00441-004-1049-5 |
| [35] |
TRAUT W, NIIMI T, IKEO K, SAHARA K. Phylogeny of the sex-determining gene sex-lethal in insects. Genome, 2006, 49(3):254-262.
doi: 10.1139/g05-107 |
| [36] |
FUJII T, SHIMADA T. Sex determination in the silkworm, Bombyx mori: A female determinant on the W chromosome and the sex-determining gene cascade. Seminars in Cell and Developmental Biology, 2007, 18(3):379-388.
doi: 10.1016/j.semcdb.2007.02.008 |
| [37] |
TANG W Q, YU L Y, HE W Y, YANG G, KE F S, BAXTER S W, YOU S J, DOUGLAS C J, YOU M S. DBM-DB: The diamondback moth genome database. Database, 2014, 2014: bat087.
doi: 10.1093/database/bat087 |
| [38] |
CHEN W, DONG Y H, SAQIB H S A, VASSEUR L, ZHOU W W, ZHENG L, LAI Y F, MA X L, LIN L Y, XU X J, BAI J L, HE W Y, YOU M S. Functions of duplicated glucosinolate sulfatases in the development and host adaptation of Plutella xylostella. Insect Biochemistry and Molecular Biology, 2020, 119:103316.
doi: 10.1016/j.ibmb.2020.103316 |
| [39] |
PING X L, SUN B F, WANG L, XIAO W, YANG X, WANG W J, ADHIKARI S, SHI Y, LV Y, CHEN Y S, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Research, 2014, 24(2):177-189.
doi: 10.1038/cr.2014.3 |
| [40] |
FUKUSUMI Y, NARUSE C, ASANO M. WTAP is required for differentiation of endoderm and mesoderm in the mouse embryo. Developmental Dynamics, 2008, 237(3):618-629.
doi: 10.1002/dvdy.v237:3 |
| [41] | HORIUCHI K, UMETANI M, MINAMI T, OKAYAMA H, TAKADA S, YAMAMOTO M, ABURATANI H, REID P C, HOUSMAN D E, HAMAKUBO T, KODAMA T. Wilms’ tumor 1-associating protein regulates G2/M transition through stabilization of cyclin A2 mRNA. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(46):17278-17283. |
| [42] |
NARUSE C, FUKUSUMI Y, KAKIUCHI D, ASANO M. A novel gene trapping for identifying genes expressed under the control of specific transcription factors. Biochemical and Biophysical Research Communications, 2007, 361(1):109-115.
doi: 10.1016/j.bbrc.2007.06.161 |
| [43] | HONGAY C F, ORR-WEAVER T L. Drosophila inducer of MEiosis 4 (IME4) is required for Notch signaling during oogenesis. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(36):14855-14860. |
| [44] |
KAN L J, GROZHIK A V, VEDANAYAGAM J, PATIL D P, PANG N, LIM K S, HUANG Y C, JOSEPH B, LIN C J, DESPIC V, et al. The m6A pathway facilitates sex determination in Drosophila. Nature Communications, 2017, 8:15737.
doi: 10.1038/ncomms15737 |
| [45] |
SINGH A, BUEHNER N A, LIN H, BARANOWSKI K J, FINDLAY G D, WOLFNER M F. Long-term interaction betweenDrosophila sperm and sex peptide is mediated by other seminal proteins that bind only transiently to sperm. Insect Biochemistry and Molecular Biology, 2018, 102:43-51.
doi: 10.1016/j.ibmb.2018.09.004 |
| [46] |
SLOAN N S, LOVEGROVE M, SIMMONS L W. Social manipulation of sperm competition intensity reduces seminal fluid gene expression. Biology Letters, 2018, 14(1):20170659.
doi: 10.1098/rsbl.2017.0659 |
| [47] |
WIGBY S, SIROT L K, LINKLATER J R, BUEHNER N, CALBOLI F C F, BRETMAN A, WOLFNER M F, CHAPMAN T. Seminal fluid protein allocation and male reproductive success. Current Biology, 2009, 19(9):751-757.
doi: 10.1016/j.cub.2009.03.036 |
| [48] | SIMMONS L W, LOVEGROVE M. Socially cued seminal fluid gene expression mediates responses in ejaculate quality to sperm competition risk. Proceedings of the Royal Society B: Biological Sciences, 2017, 284(1861):20171486. |
| [49] |
NEUBAUM D M, WOLFNER M F. Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics, 1999, 153(2):845-857.
doi: 10.1093/genetics/153.2.845 |
| [50] |
SIROT L K, BUEHNER N A, FIUMERA A C, WOLFNER M F. Seminal fluid protein depletion and replenishment in the fruit fly, Drosophila melanogaster: An ELISA-based method for tracking individual ejaculates. Behavioral Ecology and Sociobiology, 2009, 63(10):1505-1513.
doi: 10.1007/s00265-009-0806-6 |
| [51] |
GRANADINO B, SAN JUÁN A B, SÁNCHEZ L. The gene fl(2)d is required for various Sxl-controlled processes in Drosophila females. Roux’s Archives of Developmental Biology, 1991, 200(3):172-176.
doi: 10.1007/BF00190237 |
| [52] |
BOURTZIS K, LEES R S, HENDRICHS J, VREYSEN M J B. More than one rabbit out of the hat: Radiation, transgenic and symbiont- based approaches for sustainable management of mosquito and tsetse fly populations. Acta Tropica, 2016, 157:115-130.
doi: 10.1016/j.actatropica.2016.01.009 |
| [53] |
FLORES H A, O’NEILL S L. Controlling vector-borne diseases by releasing modified mosquitoes. Nature Reviews. Microbiology, 2018, 16(8):508-518.
doi: 10.1038/s41579-018-0025-0 |
| [54] |
MEZA J S, UL HAQ I, VREYSEN M J B, BOURTZIS K, KYRITSIS G A, CÁCERES C. Comparison of classical and transgenic genetic sexing strains of Mediterranean fruit fly (Diptera: Tephritidae) for application of the sterile insect technique. PLoS ONE, 2018, 13(12):e0208880.
doi: 10.1371/journal.pone.0208880 |
| [55] | ZHANG Z J, NIU B L, JI D F, LI M W, LI K, JAMES A A, TAN A J, HUANG Y P. Silkworm genetic sexing through W chromosome- linked, targeted gene integration. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(35):8752-8756. |
| [56] | GANTZ V M, JASINSKIENE N, TATARENKOVA O, FAZEKAS A, MACIAS V M, BIER E, JAMES A A. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(49):E6736-6743. |
| [57] |
HAMMOND A, GALIZI R, KYROU K, SIMONI A, SINISCALCHI C, KATSANOS D, GRIBBLE M, BAKER D, MAROIS E, RUSSELL S, BURT A, WINDBICHLER N, CRISANTI A, NOLAN T. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nature Biotechnology, 2016, 34(1):78-83.
doi: 10.1038/nbt.3439 |
| [1] | 尹飞,李振宇,SAMINA Shabbir,林庆胜. P450基因在氯虫苯甲酰胺不同抗性品系小菜蛾中的表达及功能分析[J]. 中国农业科学, 2022, 55(13): 2562-2571. |
| [2] | 孙小芳,刘敏,潘婷敏,龚国淑. 四川玉米小斑病菌交配型组成与育性分析[J]. 中国农业科学, 2021, 54(12): 2547-2558. |
| [3] | 张井勇,闫昊,彭宝,张春宝,李慧,王鹏年,丁孝羊,林春晶,孙寰,赵丽梅,张伟. 大豆RN型细胞质雄性不育系雌性育性对异交率的影响[J]. 中国农业科学, 2019, 52(8): 1324-1333. |
| [4] | 于海龙,李志远,杨丽梅,刘玉梅,庄木,吕红豪,李占省,方智远,张扬勇. 芥蓝BC3代Ogura CMS育性恢复材料的创制及Rfo基因传递和背景分析[J]. 中国农业科学, 2018, 51(9): 1746-1757. |
| [5] | 王成花,孙诗晴,徐巨龙,赵小龙,薛超彬. 抗氟苯虫酰胺小菜蛾差异表达基因及其通路[J]. 中国农业科学, 2018, 51(11): 2106-2115. |
| [6] | 魏大勇,谭传东,崔艺馨,吴道明,李加纳,梅家琴,钱伟. 甘蓝型油菜polCMS育性恢复位点的全基因组关联分析[J]. 中国农业科学, 2017, 50(5): 802-810. |
| [7] | 孔畅仪1, 王桂荣2, 刘杨2, 严善春1. 小菜蛾三个普通气味受体基因的克隆及表达谱[J]. 中国农业科学, 2014, 47(9): 1735-1742. |
| [8] | 祝丽英, 陈景堂, 黄亚群, 赵永锋, 宋占权. 一个玉米细胞质雄性不育系的鉴定及遗传分析[J]. 中国农业科学, 2012, 45(9): 1676-1684. |
| [9] | 王健立, 李洪刚, 冯志国, 郑长英. 西花蓟马与烟蓟马在紫甘蓝上的种间竞争[J]. 中国农业科学, 2011, 44(24): 5006-5012. |
| [10] | 黎相广, 严会超, 曾佩玲, 张德祥, 王修启. 温氏土鸡和白洛克鸡胚小肠EAATs mRNA表达差异及发育性变化[J]. 中国农业科学, 2011, 44(21): 4474-4480. |
| [11] | 郭艳萍,位芳,张改生,程海刚,宋瑜龙,王青,牛娜,马守才,李红霞 . 1BL/1RS小麦的分子和细胞学鉴定及黏类CMS育性恢复区域分布的分析[J]. 中国农业科学, 2010, 43(14): 2839-2847 . |
| [12] | 李罗江,茹振刚,高庆荣,姜 辉,郭凤芝,吴世文,孙 哲 . BNS小麦的雄性不育性及其温光特性[J]. 中国农业科学, 2009, 42(9): 3019-3027 . |
| [13] | 程罗根,于 光,李忠英. 小菜蛾nAChR靶标敏感性与沙蚕毒素类药物的抗性关系[J]. 中国农业科学, 2008, 41(4): 1048-1052 . |
| [14] | 曾勇庆,王根林,魏述东,王林云,王 刚,包新见,曹洪防,石景胜. 莱芜猪肌肉胶原蛋白的发育性变化及其与肉质的相关性分析[J]. 中国农业科学, 2008, 41(2): 619-624 . |
| [15] | . 苦瓜叶提取物对小菜蛾的拒食活性及有效成分研究[J]. 中国农业科学, 2008, 41(10): 3116-3122 . |
|
||