













中国农业科学 ›› 2022, Vol. 55 ›› Issue (6): 1253-1262.doi: 10.3864/j.issn.0578-1752.2022.06.016
• 畜牧·兽医·资源昆虫 • 上一篇
龙艳碧1( ),吴云飞1,张倩1,陈鹏1,2,潘敏慧1,2(
),吴云飞1,张倩1,陈鹏1,2,潘敏慧1,2( )
)
收稿日期:2021-09-10
接受日期:2021-10-20
出版日期:2022-03-16
发布日期:2022-03-25
通讯作者:
潘敏慧
作者简介:龙艳碧,E-mail: 基金资助:
LONG YanBi1( ),WU YunFei1,ZHANG Qian1,CHEN Peng1,2,PAN MinHui1,2(
),WU YunFei1,ZHANG Qian1,CHEN Peng1,2,PAN MinHui1,2( )
)
Received:2021-09-10
Accepted:2021-10-20
Online:2022-03-16
Published:2022-03-25
Contact:
MinHui PAN
摘要:
【目的】HSP90是热休克蛋白家族的成员之一,在昆虫的抗逆性和变态发育中发挥重要作用,已有研究表明HSP90能够促进家蚕核型多角体病毒(Bombyx mori nucleopolyhedrovirus,BmNPV)的增殖,但作用机制尚不清楚。本研究通过鉴定BmHSP90的相互作用蛋白,为其促进BmNPV增殖的作用机制解析提供参考。【方法】构建连接在pIZ/V5-His的BmHSP90HA真核过表达载体,在家蚕BmN-SWU1细胞中转染48 h后,感染BmNPV继续培养48 h后收集蛋白,保留总蛋白后将这些蛋白均分两管,进行免疫共沉淀,分别用抗HA抗体和IgG抗体钓取相互作用蛋白,对蛋白胶进行硝酸银染色后,获取差异条带并做质谱分析,将质谱结果与信息分析结合进行候选互作蛋白筛选,并克隆鉴定互作蛋白。通过免疫荧光验证HSP90与互作蛋白的共定位情况,并进一步采用免疫共沉淀试验确定其是否存在相互作用关系。【结果】硝酸银染色结果显示,试验组与对照组在90、70和60 kD附近处存在差异条带,并验证了90 kD处的差异条带为诱饵蛋白;将另外两条差异带进行质谱分析,共鉴定到7个候选相互作用蛋白,通过分析选择其中两个候选蛋白作后续研究,分别为Tubulin-specific chaperone E(Tbce)和Golgin subfamily A member 5(Golga5)。BmTbce的最大开放阅读框长度为1 728 bp,编码576个氨基酸,BmGolga5的最大开放阅读框长度为1 854 bp,编码618个氨基酸;同源比对和系统进化树显示BmTbce的微管结合结构域(cytoskeleton-associated protein-glycine-rich,CAP-Gly)位于N端且在不同物种之间保守性较高,BmGolga5的跨膜区(transmembrane domain,TMD)位于C端,也较为保守;荧光共定位显示BmHSP90与BmTbce和BmGolga5在细胞质中发生共定位,并进一步通过免疫共沉淀证明BmHSP90HA和BmTbceFlag、BmHSP90HA和BmGolga5Flag具有相互作用关系。【结论】经过筛选与鉴定,在BmNPV感染家蚕细胞的过程中,与家蚕热休克蛋白HSP90发生相互作用的蛋白为BmTbce和BmGolga5。
龙艳碧,吴云飞,张倩,陈鹏,潘敏慧. 家蚕热休克蛋白HSP90相互作用蛋白的筛选与鉴定[J]. 中国农业科学, 2022, 55(6): 1253-1262.
LONG YanBi,WU YunFei,ZHANG Qian,CHEN Peng,PAN MinHui. Screening and Identification of HSP90 Interacting Proteins in Silkworm (Bombyx mori)[J]. Scientia Agricultura Sinica, 2022, 55(6): 1253-1262.
表1
本研究所用引物"
| 引物名称Primer name | 引物序列Primer sequence |
|---|---|
| BmHSP90HA-Sac I-F | 5′-CGAGCTCATGTACCCATACGATGTTCCAGATTACGCTATGCCGGAAGAAATGGAGACA-3′ |
| BmHSP90HA-Sac II-R | 5′-TCCCCGCGGGGATTAAGCGTAATCTGGAACATCGTATGGGTAATCAACCTCCTCCATGCGA-3′ |
| BmGolga5Flag-Xho I-F | 5′-CCGCTCGAGCATGGATTACAAGGATGACGACGATAAGATGGCTTGGTTTGCGGACTT-3′ |
| BmGolga5Flag-Sac II-R | 5′-TCCCCGCGGCTTATCGTCGTCATCCTTGTAATCTGACTTCATCGCTCTCGTGATAT-3′ |
| BmTbceFlag-BamH I-F | 5′-CGGGATCCATGGATTACAAGGATGACGACGATAAGATGAGTAAAGTTTTTGTGCAAG-3′ |
| BmTbceFlag-Not I-R | 5′-GAATGCGGCCGCTCTTATCGTCGTCATCCTTGTAATCTCTGAATTTAACTAGAATAACATCG-3′ |
表2
BmHSP90免疫共沉淀候选蛋白"
| 蛋白ID Protein ID | 描述 Description | 分子量 Molecular weight (kD) | 功能 Function |
|---|---|---|---|
| XP_012553114 | Serine/threonine-protein phosphatase 5 (PP5) | 53.90 | 信号转导机制、能量代谢 Signal transduction mechanisms, energy metabolism |
| NP_001268820 | Juvenile hormone esterase-like precursor | 58.41 | 控制细胞周期、细胞分裂 Cell cycle control, cell division |
| XP_004923154 | Tubulin-specific chaperone E | 58.52 | 控制细胞周期、细胞分裂、染色体分配 Cell cycle control, cell division, chromosome partitioning |
| XP_004923957 | 60 kD heat shock protein (HSP60) | 62.91 | 翻译后修饰、蛋白质折叠、伴侣 Posttranslational modification, protein turnover, chaperones |
| XP_012550337 | Golgin subfamily A member 5 | 67.87 | 碳水化合物的运输和代谢 Carbohydrate transport and metabolism |
| XP_012545391 | SAM and SH3 domain-containing protein 1-like | 70.73 | 信号转导机制Signal transduction mechanisms |
| NP_001036892 | Heat shock cogn protein | 71.39 | 翻译后修饰、蛋白质折叠、伴侣 Posttranslational modification, protein turnover, chaperones |

图2
家蚕Tbce与其他物种的同源基因在CAP-Gly结构域处的序列比对 蓝色框区的氨基酸残基表示在所有比对物种中,有≥70%是一致的The amino acid residues in the blue box indicate that ≥70% of all the compared species are identical。斜纹夜蛾Spodoptera litura (XP_022828735.1);棉铃虫Helicoverpa armigera (XP_021199983.1);帝王蝶 Danaus plexippus (OWR53855.1);金凤蝶Papilio machaon (KPJ17724.1);家蚕Bombyx mori (XP_004923154.1);黑腹果蝇Drosophila melanogaster (NP_610197.2);人Homo sapiens (NP_001072983.1 );非洲爪蟾Xenopus laevis (NP_001088530.1);斑马鱼Danio rerio (NP_001035078.2);芜菁叶蜂Athalia rosae (XP_012251255.1)"
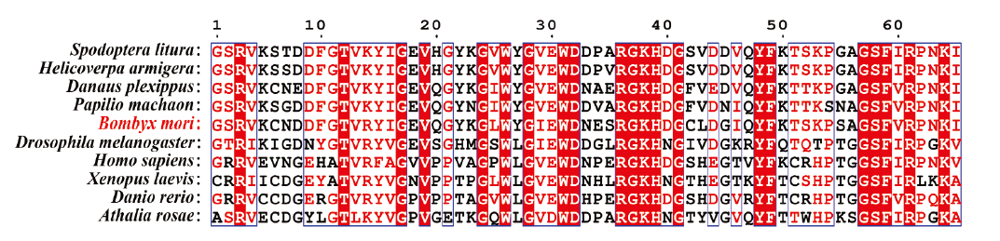

图4
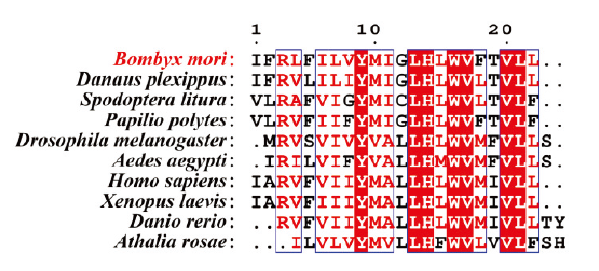
家蚕Golga5与其他物种的同源基因在跨膜区的序列比对 蓝色框区的氨基酸残基表示在所有比对物种中,有≥75%是一致的The amino acid residues in the blue box indicate that ≥75% of all the compared species are identical。家蚕Bombyx mori (XP_021207081.1);帝王蝶Danaus plexippus (XP_032522464.1);斜纹夜蛾Spodoptera litura (XP_022815171.1);玉带凤蝶Papilio polytes (XP_013139496.1);黑腹果蝇Drosophila melanogaster (NP_651250.2);埃及伊蚊Aedes aegypti (XP_021706120.1);人 Homo sapiens (AAD09753.1);非洲爪蟾Xenopus laevis (NP_001085841.1);斑马鱼Danio rerio (NP_998576.1);芜菁叶蜂Athalia rosae (XP_012252705.1)"
| [1] |
RITOSSA F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia, 1962,18:571-573.
doi: 10.1007/BF02172188 |
| [2] |
TISSIÉRES A, MITCHELL H K, TRACY U M. Protein synthesis in salivary glands of Drosophila melanogaster: Relation to chromosome puffs. Journal of Molecular Biology, 1974,84(3):389-398.
doi: 10.1016/0022-2836(74)90447-1 |
| [3] |
LANDAIS I, POMMET J M, MITA K, NOHATA J, GIMENEZ S, FOURNIER P, DEVAUCHELLE G, DUONOR-CERUTTI M, OGLIASTRO M. Characterization of the cDNA encoding the 90 kDa heat-shock protein in the Lepidoptera Bombyx mori and Spodoptera frugiperda. Gene, 2001,271(2):223-231.
doi: 10.1016/S0378-1119(01)00523-6 |
| [4] |
RICHTER K, HASLBECK M, BUCHNER J. The heat shock response: Life on the verge of death. Molecular Cell, 2010,40(2):253-266.
doi: 10.1016/j.molcel.2010.10.006 |
| [5] |
MOGK A, BUKAU B. Role of sHsps in organizing cytosolic protein aggregation and disaggregation. Cell Stress and Chaperones, 2017,22(4):493-502.
doi: 10.1007/s12192-017-0762-4 |
| [6] |
BAR-LAVAN Y, SHEMESH N, BEN-ZVI A. Chaperone families and interactions in metazoa. Essays in Biochemistry, 2016,60(2):237-253.
doi: 10.1042/EBC20160004 |
| [7] |
DALIDOWSKA I, GAZI O, SULEJCZAK D, PRZYBYLSKI M, BIEGANOWSKI P. Heat shock protein 90 chaperones E1A early protein of adenovirus 5 and is essential for replication of the virus. International Journal of Molecular Sciences, 2021,22(4):2020.
doi: 10.3390/ijms22042020 |
| [8] | ZHANG Y, ZHANG Y A, TU J. Hsp90 is required for snakehead vesiculovirus replication via stabilization the viral L protein. Journal of Virology, 2021,95(16):e00594-21. |
| [9] |
JING L, BUCHNER J. Structure, function and regulation of the Hsp90 machinery. Biomedical Journal, 2013,36(3):106-117.
doi: 10.4103/2319-4170.113230 |
| [10] |
WU P, SHANG Q, HUANG H, ZHANG S, ZHONG J, HOU Q, GUO X. Quantitative proteomics analysis provides insight into the biological role of Hsp90 in BmNPV infection in Bombyx mori. Journal of Proteomics, 2019,203:103379.
doi: 10.1016/j.jprot.2019.103379 |
| [11] |
TSOU Y L, LIN Y W, CHANG H W, LIN H Y, SHAO H Y, YU S L, LIU C C, CHITRA E, SIA C, CHOW Y H. Heat shock protein 90: Role in enterovirus 71 entry and assembly and potential target for therapy. PLoS ONE, 2013,8(10):e77133.
doi: 10.1371/journal.pone.0077133 |
| [12] |
IYER K, CHAND K, MITRA A, TRIVEDI J, MITRA D. Diversity in heat shock protein families: Functional implications in virus infection with a comprehensive insight of their role in the HIV-1 life cycle. Cell Stress and Chaperones, 2021,26(5):743-768.
doi: 10.1007/s12192-021-01223-3 |
| [13] |
MIYATA Y, YAHARA I. p53-independent association between SV40 large T antigen and the major cytosolic heat shock protein, HSP90. Oncogene, 2000,19(11):1477-1484.
doi: 10.1038/sj.onc.1203475 |
| [14] |
MOMOSE F, NAITO T, YANO K, SUGIMOTO S, MORIKAWA Y, NAGATA K. Identification of Hsp90 as a stimulatory host factor involved in influenza virus RNA synthesis. The Journal of Biological Chemistry, 2002,277(47):45306-45314.
doi: 10.1074/jbc.M206822200 |
| [15] |
WYLER E, MOSBAUER K, FRANKE V, DIAG A, GOTTULA L T, ARSIE R, KLIRONOMOS F, KOPPSTEIN D, HONZKE K, AYOUB S, et al. Transcriptomic profiling of SARS-CoV-2 infected human cell lines identifies HSP90 as target for COVID-19 therapy. iScience, 2021,24(3):102151.
doi: 10.1016/j.isci.2021.102151 |
| [16] | 张凤霞. 棉铃虫Hsp90、胸腺素和丝氨酸蛋白酶抑制因子在发育中的表达模式与激素调控[D]. 济南: 山东大学, 2010. |
| ZHANG F X. Expression patterns and hormonal regulation of Hsp90, thymosin and serine protease inhibitors in the development of Helicoverpa armigera[D]. Ji’nan: Shandong University, 2010. (in Chinese) | |
| [17] |
CHEN B, WAGNER A. Hsp90 is important for fecundity, longevity, and buffering of cryptic deleterious variation in wild fly populations. BMC Evolutionary Biology, 2012,12:25.
doi: 10.1186/1471-2148-12-25 |
| [18] |
MCCLELLAN A J, XIA Y, DEUTSCHBAUER A M, DAVIS R W, GERSTEIN M, FRYDMAN J. Diverse cellular functions of the Hsp90 molecular chaperone uncovered using systems approaches. Cell, 2007,131(1):121-135.
doi: 10.1016/j.cell.2007.07.036 |
| [19] |
SHANG Q, WU P, HUANG H L, ZHANG S L, TANG X D, GUO X J. Inhibition of heat shock protein 90 suppresses Bombyx mori nucleopolyhedrovirus replication in B. mori. Insect Molecular Biology, 2020,29(2):205-213.
doi: 10.1111/imb.v29.2 |
| [20] |
PAN M H, CAI X J, LIU M, LV J, TANG H, TAN J, LU C. Establishment and characterization of an ovarian cell line of the silkworm, Bombyx mori. Tissue and Cell, 2010,42(1):42-46.
doi: 10.1016/j.tice.2009.07.002 |
| [21] |
BASCOM R A, SRINIVASAN S, NUSSBAUM R L. Identification and characterization of golgin-84, a novel Golgi integral membrane protein with a cytoplasmic coiled-coil domain. The Journal of Biological Chemistry, 1999,274(5):2953-2962.
doi: 10.1074/jbc.274.5.2953 |
| [22] |
SATOH A, WANG Y, MALSAM J, BEARD M B, WARREN G. Golgin-84 is a rab1 binding partner involved in Golgi structure. Traffic, 2003,4(3):153-161.
doi: 10.1034/j.1600-0854.2003.00103.x |
| [23] |
DIAO A, RAHMAN D, PAPPIN D J, LUCOCQ J, LOWE M. The coiled-coil membrane protein golgin-84 is a novel rab effector required for Golgi ribbon formation. The Journal of Cell Biology, 2003,160(2):201-212.
doi: 10.1083/jcb.200207045 |
| [24] |
PINOT M, GOUD B, MANNEVILLE J B. Physical aspects of COPI vesicle formation. Molecular Membrane Biology, 2010,27(8):428-442.
doi: 10.3109/09687688.2010.510485 |
| [25] |
SOHDA M, MISUMI Y, YAMAMOTO A, NAKAMURA N, OGATA S, SAKISAKA S, HIROSE S, IKEHARA Y, ODA K. Interaction of Golgin-84 with the COG complex mediates the intra-Golgi retrograde transport. Traffic, 2010,11(12):1552-1566.
doi: 10.1111/tra.2010.11.issue-12 |
| [26] |
TONGMUANG N, YASAMUT U, SONGPRAKHON P, DECHTAWEWAT T, MALAKAR S, NOISAKRAN S, YENCHITSOMANUS P T, LIMJINDAPORN T. Coat protein complex I facilitates dengue virus production. Virus Research, 2018,250:13-20.
doi: 10.1016/j.virusres.2018.03.021 |
| [27] |
LIMJINDAPORN T, WONGWIWAT W, NOISAKRAN S, SRISAWAT C, NETSAWANG J, PUTTIKHUNT C, KASINRERK W, AVIRUTNAN P, THIEMMECA S, SRIBURI R, SITTISOMBUT N, MALASIT P, YENCHITSOMANUS P T. Interaction of dengue virus envelope protein with endoplasmic reticulum-resident chaperones facilitates dengue virus production. Biochemical and Biophysical Research Communications, 2009,379(2):196-200.
doi: 10.1016/j.bbrc.2008.12.070 |
| [28] |
HOWE C, GARSTKA M, AL-BALUSHI M, GHANEM E, ANTONIOU A N, FRITZSCHE S, JANKEVICIUS G, KONTOULI N, SCHNEEWEISS C, WILLIAMS A, ELLIOTT T, SPRINGER S. Calreticulin-dependent recycling in the early secretory pathway mediates optimal peptide loading of MHC class I molecules. The EMBO Journal, 2009,28(23):3730-3744.
doi: 10.1038/emboj.2009.296 |
| [29] |
WU Y, DING Y, ZHENG X, LIAO K. The molecular chaperone Hsp90 maintains Golgi organization and vesicular trafficking by regulating microtubule stability. Journal of Molecular Cell Biology, 2020,12(6):448-461.
doi: 10.1093/jmcb/mjz093 |
| [30] | 高囡囡, 鲍岚. 微管蛋白的翻译后修饰及功能研究. 生命科学, 2015,27(3):363-373. |
| GAO N N, BAO L. Post-translational modification and function of tubulin. Chinese Bulletin of Life Sciences, 2015,27(3):363-373. (in Chinese) | |
| [31] |
TIAN G, HUANG Y, ROMMELAERE H, VANDEKERCKHOVE J, AMPE C, COWAN N J. Pathway leading to correctly folded β-tubulin. Cell, 1996,86(2):287-296.
doi: 10.1016/S0092-8674(00)80100-2 |
| [32] |
JIN S, PAN L, LIU Z, WANG Q, XU Z, ZHANG Y Q. Drosophila tubulin-specific chaperone E functions at neuromuscular synapses and is required for microtubule network formation. Development, 2009,136(9):1571-1581.
doi: 10.1242/dev.029983 |
| [33] |
METIVIER M, GALLAUD E, THOMAS A, PASCAL A, GAGNE J P, POIRIER G G, CHRETIEN D, GIBEAUX R, RICHARD-PARPAILLON L, BENAUD C, GIET R. Drosophila tubulin-specific chaperone E recruits tubulin around chromatin to promote mitotic spindle assembly. Current Biology, 2021,31(4):684-695.
doi: 10.1016/j.cub.2020.11.009 |
| [34] |
HYDE J L, GILLESPIE L K, MACKENZIE J M. Mouse norovirus 1 utilizes the cytoskeleton network to establish localization of the replication complex proximal to the microtubule organizing center. Journal of Virology, 2012,86(8):4110-4122.
doi: 10.1128/JVI.05784-11 |
| [35] | DIGIUSEPPE S, LUSZCZEK W, KEIFFER T R, BIENKOWSKA- HABA M, GUION L G, SAPP M J. Incoming human papillomavirus type 16 genome resides in a vesicular compartment throughout mitosis. Proceedings of the National Academy of Sciences of the United States of America, 2016,113(22):6289-6294. |
| [36] | LOFTUS M S, VERVILLE N, KEDES D H. A conserved leucine zipper motif in gammaherpesvirus ORF52 is critical for distinct microtubule rearrangements. Journal of Virology, 2017,91(17):e00304-17. |
| [37] |
CHEN S, LIU M, HUANG H, LI B, ZHAO H, FENG X Q, ZHAO H P. Heat stress-induced multiple multipolar divisions of human cancer cells. Cells, 2019,8(8):888.
doi: 10.3390/cells8080888 |
| [38] |
LI S, WANG Y, HOU D, GUAN Z, SHEN S, PENG K, DENG F, CHEN X, HU Z, WANG H, WANG M. Host factor heat-shock protein 90 contributes to baculovirus budded virus morphogenesis via facilitating nuclear actin polymerization. Virology, 2019,535:200-209.
doi: 10.1016/j.virol.2019.07.006 |
| [1] | 陈鹏,包希艳,康涛涛,董战旗,朱艳,潘敏慧,鲁成. 家蚕IAP互作蛋白的筛选、鉴定及其对BmNPV增殖的影响[J]. 中国农业科学, 2019, 52(3): 558-567. |
| [2] | 董战旗,蒋亚明,潘敏慧. 家蚕热休克蛋白HSP60相互作用蛋白筛选与鉴定[J]. 中国农业科学, 2019, 52(2): 376-384. |
| [3] | 易敏,吕青,刘柯柯,王礼君,吴玉娇,周泽扬,龙梦娴. 家蚕微孢子虫极管蛋白2(NbPTP2)的表达、纯化和定位特征[J]. 中国农业科学, 2019, 52(10): 1830-1838. |
| [4] | 张奎,李重阳,苏晶晶,谈娟,徐曼,崔红娟. 家蚕整合素β2的表达、纯化及其免疫功能[J]. 中国农业科学, 2019, 52(1): 181-190. |
| [5] | 张奎,潘光照,苏晶晶,谈娟,徐曼,李钰添,崔红娟. 家蚕glial cell missing (BmGcm)基因鉴定、表达、亚细胞定位和功能[J]. 中国农业科学, 2018, 51(7): 1401-1411. |
| [6] | 王菲,李显扬,化晓婷,夏庆友. 家蚕抗BmNPV细胞因子的筛选和分析[J]. 中国农业科学, 2018, 51(4): 789-799. |
| [7] | 胡杰,王鑫怡,王菲. 家蚕BmCaspase-8-Like(BmCasp8L)的免疫负调控功能[J]. 中国农业科学, 2018, 51(21): 4188-4196. |
| [8] | 龙定沛,郝占章,向仲怀,赵爱春. 家蚕安全转基因技术研究现状与展望[J]. 中国农业科学, 2018, 51(2): 363-373. |
| [9] | 张艳,董照明,席星航,张晓璐,叶林,郭凯雨,夏庆友,赵萍. 家蚕脱胶蚕丝的蛋白组成成分[J]. 中国农业科学, 2018, 51(11): 2216-2224. |
| [10] | 张薇薇,董照明,张艳,张晓璐,张守亚,赵萍. 家蚕表皮蛋白BmCPAP3-G的表达特征及其与几丁质的结合特性[J]. 中国农业科学, 2017, 50(9): 1723-1733. |
| [11] | 张倩,刘太行,董小龙,吴云飞,杨基贵,周亮,潘彩霞,潘敏慧. 家蚕CDK11与RNPS1和9G8相互作用的鉴定[J]. 中国农业科学, 2017, 50(22): 4398-4407. |
| [12] | 蒋亚明,董战旗,陈婷婷,胡楠,董非凡,黄亮,唐良彤,潘敏慧. 杆状病毒LEF-11蛋白自身相互作用关键区域的鉴定[J]. 中国农业科学, 2017, 50(20): 4028-4035. |
| [13] | 潘光照,张奎,李重阳,赵羽卒,申利,徐曼,苏晶晶,林西,崔红娟. 家蚕组织蛋白酶L(BmCathepsin L)基因鉴定、表达及其功能分析[J]. 中国农业科学, 2017, 50(16): 3236-3246. |
| [14] | 何华伟,王叶菁,侯丽,李瑜,位曙光,赵朋,蒋文超,赵萍. 家蚕碱性磷酸酶原核表达纯化、结构与活性分析[J]. 中国农业科学, 2017, 50(14): 2837-2850. |
| [15] | 高瑞,李春林,童晓玲,曹明亚,石美宁,徐安英,鲁成,代方银. 分子连锁分析探讨家蚕高抗BmNPV品系的抗性遗传基础[J]. 中国农业科学, 2017, 50(1): 195-204. |
|
||